Chapter 4
ETHANOL PRODUCTION - GENERAL DISCUSSION
RAW MATERIALS
Ethyl alcohol may be made by the fermentation process from three basic types of raw materials, called "feedstock".
The three basic types of feedstock are:
(1 ) SACCHARINE (sugar containing) materials in which the carbohydrate (the actual substance from which the alcohol is made) is present in the form of simple, directly fermentable six and twelve carbon sugar molecules such as glucose, fructose, and maltose. Such materials include sugar cane, sugar beets, fruit (fresh or dried), citrus molasses, cane sorghum, whey and skim milk.
(2) STARCHY MATERIALS that contain more complex carbohydrates such as starch and inulin that can be broken down into the simpler six and twelve carbon sugars by hydrolysis with acid or by the action of enzymes in a process called malting. Such materials include corn, grain sorghum, barley, wheat, potatoes, sweet potatoes, jerusalem artichokes, cacti, manioc, arrowroot, and so on.
(3) CELLULOSE MATERIALS such as wood, wood waste, paper, straw, corn stalks, corn cobs, cotton, etc., which contain material that can be hydrolyzed with acid, enzymes or otherwise converted into fermentable sugars called glucose.
MANUFACTURING STEPS
Certain materials require less processing than others. Generally, small scale production is easiest (and most economical in terms of labor and energy consumption) from the saccharine materials. However, starchy materials usually produce the most alcohol on a weight/weight basis, and cellulose materials are the cheapest.
Manufacturing alcohol from saccharine feedstocks generally requires: (1) extraction or crushing to make the sugars available to the yeast enzymes during fermentation: (2) dilution. which is only required with certain materials; (3) fermentation; and (4) distillation. Starchy materials require the steps of: (1) milling to free the starchy material from, for example, grain kernels; (2) dilution; (3) cooking to dissolve and "gelatinize" the starch; and (4) conversion of the starch to fermentable sugars by malting, enzymes, or acid hydrolysis in addition to the steps of fermentation and distillation. Cellulose materials are similar to starchy materials in that they must be converted prior to fermentation.
PROCESS DESIGN
There are a great many variables in the manufacture of ethanol. Even materials from the same basic group can require radically different processing. The following chapters cover the individual manufacturing steps for processing each of the three main groups of feedstock. In addition, Chapter 10 contains "recipes" and individual processing requirements for specific materials from each of the three groups.
The reader is urged to study all of the information presented before attempting to choose a specific process for a material.
Chapter 5
PROCESSING STEPS COMMON TO ALL MATERIALS
DILUTION
Dilution is simply the addition of water to adjust the amount of sugar in the mash or (ultimately) the amount of alcohol in the beer. It is necessary because the yeast, used later in the fermentation process, can be killed by too great a concentration of alcohol. Also, during the mashing and conversion of starchy material, dilution is necessary to make the mash easier to stir and handle. The object of dilution is to end up with a beer as close to (but, not more than) 10% alcohol when fermentation is complete. The optimum dilution, then, is a compromise between the highest alcohol concentration and the point where the particular yeast strain being used will be killed.
Optimum dilution requirements for each material are listed in Chapter 10. A rule of thumb for an unknown material, though, is that the final alcohol concentration will be about half the sugar content prior to fermentation. To determine the amount of fermentable sugar in a mash, it is best to have the material tested by a laboratory. If this is not possible, the sugar content can be estimated with a hydrometer. The use of hydrometers and tables for converting specific gravity readings to approximate sugar content are covered later in this chapter. It should be noted that any solution being tested with a hydrometer must be filtered to remove any undissolved solids. Otherwise the readings will be inaccurate. Sugar content of a solution can also be determined with the use of an optical instrument called a sugar refractometer. These devices, however, cost several hundred dollars.
Since the use of a hydrometer to measure sugar content of a mash is, at best, an approximation, the amount of dilution can be "fine tuned" by measuring the alcohol content of the beer after fermentation. A hydrometer is used for this measurement also, but the readings are much more accurate. Naturally, if the alcohol content of the beer is less than the toleration level of the yeast you are using, the mash is overdiluted.
pH CONTROL
The pH is a measure of the acidity or alkalinity of an aqueous solution expressed on a scale of 1-14. Neutral is pH 7, pH 1-7 is acid, and pH 7-14 is alkaline. The pH is most conveniently measured with test papers that change color according to the pH of the solution being tested. These papers are available from swimming pool supply houses, garden shops, and laboratory supply companies.
Control of pH during the mashing and fermentation process is important for two reasons: The growth of harmful bacteria is retarded by acid solutions, and yeast will grow only in an (slightly) acid solution.
Most grain mashes have a naturally acid pH of between 5.4 and 5.6 after malting or conversion has been accomplished. Other materials, notably saccharine substances like molasses and fruit pressings, have a naturally alkaline pH and must be acidified prior to fermentation.
The principal bacterial contaminants in a distillery are those that form lactic acid. Although the production of fuel alcohol is not concerned with the taste of the product, any lactic acid formed subtracts from the yield of alcohol. The production of lactic acid and other contaminants should therefore be avoided as much as possible. The development of these micro-organisms is severely repressed at pH values under 5.0. Above 5.0 their growth is rapid. The optimum pH range then is 4.8 to 5.0. Anything below about 4.1 to 4.4 is detrimental to other (desirable) processes taking place during the mashing and fermentation. Consequently, the pH should be checked during the cooking and conversion. If it is much above 5.0, it should be reduced by the addition of acid.
The acid most commonly used is sulfuric, although any mineral acid is perfectly suitable. Hydrochloric (muriatic) acid, for example, is available from swimming pool suppliers. The acid should be added cautiously, the mash stirred, and the pH checked, because it is very important not to add too much. If you happen to add a little too much, the pH can be raised with sodium hydroxide (caustic soda) solution or with ordinary lime. But after a certain point, this is useless and the mash must be scrapped.
While adjustment during mashing is desirable, the proper pH during fermentation is absolutely essential. As soon as the pH in fermentation falls below about 4.11 the fermentation stops. If this occurs prior to complete conversion of the sugars, the yield will be low. On the other hand, yeast needs a slightly acid environment in order to grow. Consequently, the pH should be kept between 4.8 and 5.0 for optimum results.
There are two ways of adjusting pH. The first, as discussed, is the addition of acid. The second, and probably the best, is the addition of the naturally acid residues left from a previous distillation. These residues are called "stillage", and adding them to the mash is called "backslopping". Backslopping is discussed in more detail in the next section.
It should be stressed that the pH should be checked periodically during the fermentation as well as before. Certain fermentations will produce substances that alter the pH during the fermentation. Once the pH goes beyond the optimum range, attempts to salvage the process by adding acid or caustic soda do more harm than good. So keep a close watch and adjust before the pH goes out of range.
BACKSLOPPING
Backslopping, or the addition of still residues from the previous batch, has several advantages. First (as discussed in the previous section) is the adjustment of the pH to control bacteria growth. Second, the stillage provides nutrients that are needed by the yeast for rapid growth. The third reason is that the stillage provides a "buffering" action.
Grain mashes and starchy material generally provide enough nutrients for the growth of the yeast. Other materials, notably molasses and other saccharine materials, often do not. The addition of stillage can provide these nutrients where they are needed.
The buffer capacity of the mash is important. When an acid and a base are mixed together, they react violently to produce a salt. Buffering can be thought of as a barrier between the acid and the base that allows only limited contact and thus moderates the reaction. Grain mashes are generally well buffered between pH 5.0 to 6.0, poorly buffered between 4.4 and 5.07 and well buffered between 3.5 and 4.4. The addition of stillage aids in buffering the mash between 4.4 and 5.0. This provides stability and generally higher yields than mashes without stillage.
Different materials can tolerate differing amounts of backslopping. It is possible to have too much of a good thing, and too much backslopping can be detrimental. The limits for various materials are discussed in Chapter 10.
CLEANLINESS
The cleaning of fermenting tubs, pipes, and the like is extremely important. If mash and fermentation residues are allowed to accumulate, bacterial contamination will be rampant and will greatly reduce alcohol yield.
Cleaning of the mashing and fermentation apparatus is usually done with steam in commercial operations. However, in a small plant, a thorough washing with disinfectant is usually adequate. Any disinfecting cleaner can be used, but, in the interest of economy, it is best to buy formaldehyde solution from a chemical supply house. For use it should be diluted 20:1 or more. Be advised that formaldehyde is a horribly foul smelling chemical that is intensely irritating to the skin, nose and eyes. The fumes also should not be inhaled. An alternate to formaldehyde is ammonia (ammonium hydroxide) solution, but the same cautions apply.
After disinfecting with formaldehyde or ammonia, the apparatus should be thoroughly washed out with clear water. It is best to clean equipment after every batch, but in some climates and at certain times of the year when the bacteria count is low, cleaning every second or third time might be all right. In any event, at the first sign of problems, a thorough cleaning is absolutely necessary.
HYDROMETERS
Figure 5-1: Hydrometer
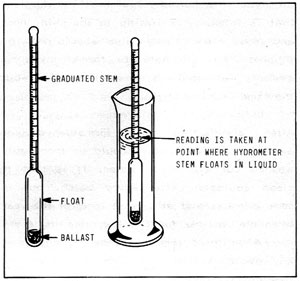
As illustrated in Figure 5-1, hydrometers are little floats with calibrated stems used to measure the specific gravity of a liquid. The most familiar example is the device used to check battery charge or anti-freeze protection.
Figure 5-2: Sugar Content vs Specific Gravity
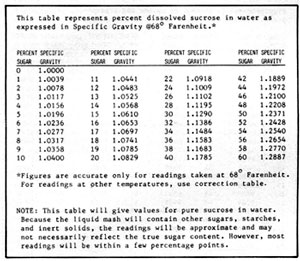
Hydrometers can be calibrated in a number of different scales, depending on their purpose. The most common calibration is for specific gravity. Water has a specific gravity of 1.000. Liquids lighter than water have specific gravities less than 1.000, and those heavier, greater than 1.000. The hydrometer can be used to measure the approximate dissolved solids in a mash or the concentration of alcohol before or after distillation. For measuring the solids dissolved in a mash the hydrometer is calibrated in degrees "Balling". One degree on this scale is equal to about 1% dissolved solids. Other hydrometers can be purchased to show alcohol content in proof or percent. To obtain accurate measurements, a set of hydrometers, each covering a small range, is better than one hydrometer covering a large range. Hydrometers can be purchased from any laboratory supply house. So that you do not have to purchase several sets, Figures 5-2 and 3 convert sugar and alcohol content to specific gravity.
Figure 5-3: Alcohol Content vs Specific Gravity
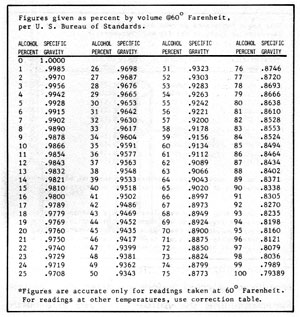
Figure 5-4: Correction Table
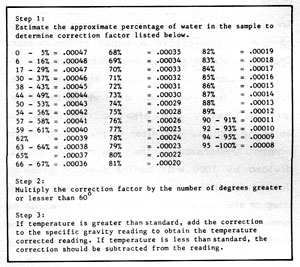
Because liquids change density with changes in temperature, corrections must be made for readings taken at temperatures other than that for which the hydrometer is calibrated. More often than not, the temperature of the solution you are testing will not be the same as the hydrometer calibration. Figure 5-4 is a correction table for non-standard temperatures. Note that this table is very accurate for determining the correction for aqueous ethanol solutions and less accurate for sugar or dissolved solids concentration.
An alternate method of determining sugar content is the use of a sugar refractometer. These instruments are available from laboratory supply houses, but are expensive. However, the readings are very accurate.
Specific gravity can also be determined by weighing exactly one liter of the liquid at the proper temperature. The weight in grams, divided by 1000 will be the specific gravity. Due to the difficulty of measuring exactly one liter of the solution being tested, the weighing method is usually not as accurate as the method using a hydrometer.
Chapter Index
Chapter 1 AN OVERVIEW
Chapter 2 BASIC FUEL THEORY
Chapter 3 UTILIZATION OF ALCOHOL FUELS
Chapter 4 ETHANOL PRODUCTION - GENERAL DISCUSSION
Chapter 5 PROCESSING STEPS COMMON TO ALL MATERIALS
Chapter 6 PROCESSING STEPS SPECIFIC TO SACCHARINE MATERIALS
Chapter 7 PROCESSING STEPS SPECIFIC TO STARCHY MATERIALS
Chapter 8 PROCESSING STEPS SPECIFIC TO CELLULOSE MATERIALS
Chapter 9 YEAST AND FERMENTATION
Chapter 10 INDIVIDUAL RAW MATERIALS
Chapter 11 DISTILLATION
Chapter 12 DRYING THE ALCOHOL
Chapter 13 MASHING AND FERMENTATION EQUIPMENT
Chapter 14 DISTILLATION EQUIPMENT
Chapter 15 SOLAR STILLS
Chapter 17 PUTTING IT ALL TOGETHER
Chapter 18 THE FUTURE
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
