Chapter 11
DISTILLATION
DISTILLATION THEORY
The object of distillation is the separation of the alcohol from the other ingredients in the beer, mostly water. In making fuel alcohol it is necessary to get all of the alcohol and water separated if the alcohol is going to be mixed with gasoline, and most of the alcohol and water separated if the alcohol is going to be burned in a converted engine. As will be seen, the purer the alcohol, the harder it is to make.
The separation of the alcohol and water by distillation is made possible by the fact that alcohol boils at about 173 degrees F. and water at 212 degrees F. When the mixture of water and alcohol is boiled, vapors with a greater concentration of alcohol will be formed and liquid with a lesser concentration of alcohol will remain behind. However, because water and alcohol do not form what is called an "ideal" mixture, the separation cannot be done in one clean step.
Figure 11 -1: SIMPLE DISTILLATION APPARATUS
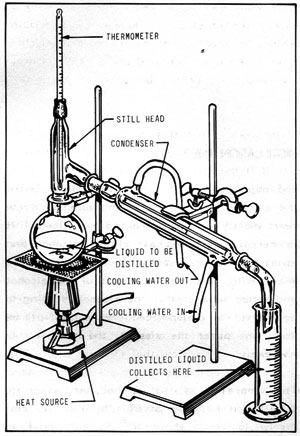
Figure 11-1 illustrates a simple distillation apparatus using laboratory-type equipment. Note that the equipment consists basically of a container for the liquid to be distilled (still pot), a heat source, and a condenser to turn the distilled vapors back into liquid form. The thermometer is necessary to monitor the temperature of the vapors.
Figure 11-2: BOILING POINT COMPOSITION for LIQUID and VAPOR PHASES
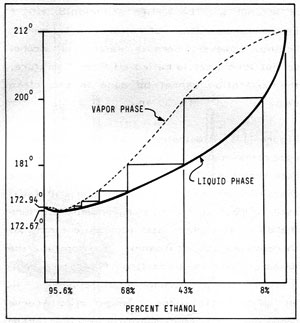
In Figure 11-2, the heavy solid curve represents the composition of the liquid phase of water/ethanol mixtures plotted against the temperature at which the mixture boils. The dotted curve represents the vapor phase. Using the apparatus illustrated, and starting with a concentration of 8% alcohol in water, the liquid will boil at about 200 degrees F. Reading across, the vapors will contain about 43% alcohol. Clearly, for fuel purposes, a purer product is needed. To this end, we must redistill the condensed vapors from the first distillation which contain 43% alcohol and 57% water. This mixture will boil at about 181 degrees F. and the vapors will contain about 68% alcohol. Each time the condensed vapors are redistilled, they will be slightly purer, but many separate distillations are needed to produce relatively pure alcohol. Fortunately, a type of distillation apparatus, called a reflux (or rectifying column), in effect, performs simultaneous distillations and will be described later.
However, with the equipment described, no matter how elaborate, the purest alcohol that can be produced is 95.6%. The remaining 4.4% water is impossible to remove because at this ratio, water and alcohol form a constant boiling mixture (called an "azetrope") whose boiling point is a fraction of a degree below that of pure alcohol , and separation by ordinary distillation is impossible. Special techniques that can remove this residual water are outlined later in Chapter 12.
THE REFLUX COLUMN
Figure 11-3 illustrates a reflux column installed on the simple apparatus described in Figure 11-1. In this laboratory version, the reflux column consists merely of a glass tube filled with packing material. In this case, the packing is short lengths of small-diameter glass tubing. The purpose of the packing is to provide as large an internal surface area as possible.
Figure 11-3: REFLUX DISTILLING APPARATUS
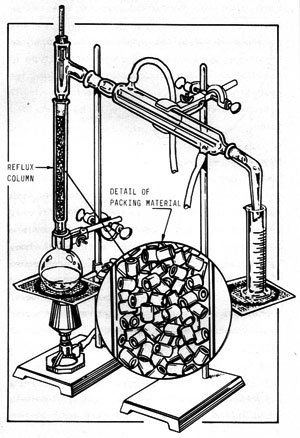
As the vapors from the still pot ascend through the column, they condense on the packing material and drip downward. Additional ascending vapors contact the descending liquid (called "reflux") and revaporize it. Thus, as the vapors slowly work their way up the column, they become richer and richer in alcohol until, when they reach the top, they are relatively pure. Meanwhile, the descending liquid is stripped of its alcohol. The overall effect is that many "distillations" are performed simultaneously and the liquid in the still pot is stripped of its alcohol in one continuous operation.
Reflux columns can be constructed to operate on either a batch or continuous basis and are described in Chapter 14.
Chapter Index
Chapter 1 AN OVERVIEW
Chapter 2 BASIC FUEL THEORY
Chapter 3 UTILIZATION OF ALCOHOL FUELS
Chapter 4 ETHANOL PRODUCTION - GENERAL DISCUSSION
Chapter 5 PROCESSING STEPS COMMON TO ALL MATERIALS
Chapter 6 PROCESSING STEPS SPECIFIC TO SACCHARINE MATERIALS
Chapter 7 PROCESSING STEPS SPECIFIC TO STARCHY MATERIALS
Chapter 8 PROCESSING STEPS SPECIFIC TO CELLULOSE MATERIALS
Chapter 9 YEAST AND FERMENTATION
Chapter 10 INDIVIDUAL RAW MATERIALS
Chapter 11 DISTILLATION
Chapter 12 DRYING THE ALCOHOL
Chapter 13 MASHING AND FERMENTATION EQUIPMENT
Chapter 14 DISTILLATION EQUIPMENT
Chapter 15 SOLAR STILLS
Chapter 17 PUTTING IT ALL TOGETHER
Chapter 18 THE FUTURE
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
