
<źVāĶāĘÉyĀ[ÉWŹ–ČÓ>
ĀúéüČŮāŐĀuéŤā√ā≠āŤÉoÉCÉIÉfÉBĀ[É[ÉčĒRóŅÉZÉ~ÉiĀ[ĀvāūāPāPĆéāRāOďķĀiďķójďķĀjā…ó\íŤāĶāńāĘā‹ā∑ĀBéQČŃé“ēŚŹWíÜĀIĀ@ŹŕāĶā≠āÕāĪāŅāÁĀB
ĀúďķĖ{äeínāŐéśāŤĎgā›āūā‹ā∆āŖāĹĀuÉoÉCÉIÉfÉBĀ[É[ÉčďķĖ{ínź}ĀvāūÉAÉbÉvāĶā‹āĶāĹĀB
ÉoÉCÉIĒRóŅ
ÉoÉCÉIÉfÉBĀ[É[ÉčĒRóŅ
é©ē™āŇćžāŃāńā›āśā§ĀIĀ@
ÉoÉCÉIÉfÉBĀ[É[ÉčĒRóŅāŐćžāŤēŻ
É}ÉCÉNāŐĀuāPíiäKĀ@ÉAÉčÉJÉäēŻéģĀv
ÉAÉĆÉbÉNÉXāŐĀuāQíiäKĀ@ÉAÉčÉJÉä-ÉAÉčÉJÉäēŻéģĀv
ÉAÉĆÉbÉNÉXāŐĀuāQíiäKĀ@é_-ÉAÉčÉJÉäĀwFoolproofĀxēŻéģ
ĒRóŅźĽĎĘäŪāŗé©ē™āŇćžāťĀI
ćžāŤēŻ
ćĀć`ÉoÉCÉIÉfÉBĀ[É[Éčē®ĆÍ
ĒrÉKÉXíÜāŐNOxāÕĖ‚ĎŤā©ĀH
ēõéYē®ÉOÉäÉZÉäÉďāŐäąópĖ@
ÉoÉCÉIÉfÉBĀ[É[ÉčŹÓēŮŹW
ÉfÉBĀ[É[Éčā…ŹęóąāÕā†āťā©ĀH
ćžē®ā…āśāťźAē®ĖŻāŐéŻó ā∆ďŃí•
ćŇĆ„āŐédŹ„āįāÕĖAāŇźŰā§
ÉoÉCÉIÉfÉBĀ[É[ÉčĒRóŅāūégā§ā∆āęāŐämĒFéĖćÄ
źHó∆vsĒRóŅĀH
źAē®ĖŻāĽāŐā‹ā‹ĒRóŅ
ÉKÉ\ÉäÉďé‘ā…āÕÉGÉ^ÉmĀ[ÉčĒRóŅāū
ÉGÉ^ÉmĀ[ÉčŹÓēŮŹW
ÉGÉ^ÉmĀ[ÉčĒRóŅāÕÉGÉlÉčÉMĀ[āūĖ≥Ď ā…āĶāńāĘāťā©ĀH
ÉoÉCÉIĒRóŅML
ÉoÉCÉIĒRóŅāŐÉIÉďÉČÉCÉďź}ŹĎäŔĀiČpē∂Āj
ÉoÉCÉIĒRóŅā∆ĒRóŅćžāŤópčÔāŐďŁéŤźśĀiČpē∂Āj
ćuČČĀEÉZÉ~ÉiĀ[āŐā≤ąńďŗ
éĚĎĪȬĒ\ā»źHā∆ÉGÉlÉčÉMĀ[ā…ā¬āĘāńā®ėbāĶāĶā‹ā∑
| ÉgÉbÉv | |
| ÉĀÉfÉBÉAĆfćŕā∆ÉRÉĀÉďÉgŹW | |
| éŤā√ā≠āŤäťČśāŐŹ–ČÓ | |
| Ė`ĆĮā™énā‹āŃāĹĆoą‹ |
|
| ÉTÉCÉgÉ}ÉbÉv | |
| ÉvÉćÉWÉFÉNÉg | |
| ínąśāŐé©óßāūĖŕéwā∑ | |
| āĪāŐäťČśāūźiāŖāťóĚóR | |
| Rural development | |
| Fixing what's broken | |
| ďsésĒ_ČÄ | |
| äXā™źHó∆āūąÁāńénāŖāĹĀI | |
| óLč@ćōČÄāŐÉXÉXÉĀ | |
| íŽāŇēĒČģāŇČhó{āĹāŃā’āŤāŐĖžćōāūąÁāńāťēŻĖ@ | |
| ďyāūā¬ā≠āť | |
| ā∑ā◊āńāŐĆíćNāÕďyā©āÁénā‹āť | |
| Ź¨ā≥ā»Ē_ŹÍ | |
| é©óRā∆é©óßā÷āŐďĻ | |
| ÉoÉCÉIĒRóŅ | |
| ínąśāŇąÁāńāťÉGÉlÉčÉMĀ[ | |
| ĎĺózĒMÉRÉďÉć | |
| źXā∆źlāūč~ā§É\Ā[ÉČĀ[ĀEÉNÉbÉJĀ[ | |
| źXā∆ďyā∆źÖā∆ | |
| éRāŐäŽč@āÕā›āŮā»āŐäŽč@ | |
| źĘäEāŐéŪéq | |
| éŪā™ā»āĮāÍāőźHāŗā»āĶ | |
| Appropriate technology | |
| What works and fits | |
| Project vehicles | |
| The workhorses | |
| ÉCÉďÉ^Ā[ÉlÉbÉg | |
| ČĹā™āĽāŮā»ā…ČśäķďIā»āŐā©ĀH | |
| Internet interaction | |
| ēKóvā»ŹÓēŮāūíTāĶŹoā∑ā…āÕ | |
| č≥ąÁäťČś | |
| č≥ąÁäťČśāŐäąópēŻ | |
| ÉoÉCÉIĒRóŅ | |
| ĎĺózĒMÉRÉďÉć | |
| čůāęä āŇćžāťä»íPÉRÉďÉć | |
| íīŹ¨Ć^ēóóÕĒ≠ďdč@ | |
| ďdĆĻāŐāĘāÁā»āĘÉćĀ[ÉeÉNĀEÉČÉWÉI | |
| íiÉ{Ā[ÉčĒ†āŐäąópĖ@ | |
| ƶāŐéoĖÖ | |
| ĆCĒ†āŇé\āūąÁāńāť | |
| äwćZćōČÄ | |
| ďyāūā¬ā≠āť | |
| írāŗā¬ā≠āť | |
| éŤā√ā≠āŤďVĎRČŠāśāĮÉXÉvÉĆĀ[ | |
| ÉlÉbÉgāŇéQČŃāŇāęāťč≥ąÁÉvÉćÉOÉČÉÄ | |
| ÉlÉbÉgāŇégā¶āťč≥ąÁä÷ĆWŹÓēŮŹW | |
| ėAóćźś éŤā√ā≠āŤäťČśĀuÉWÉÉĀ[ÉjĀ[ĀEÉgÉDĀEÉtÉHĀ[ÉGÉoĀ[Āv http://journeytoforever.org/jp/ Āß622-0291čěďsē{ĎDąšĆS čěíOĒgí¨óXē÷č«Ā@饏ĎĒ†āUćÜ ÉLĀ[ÉXĀEÉAÉfÉBÉ\ÉďĀ@(ČpĆÍ) ēĹČÍóőĀ@(ďķĖ{ĆÍĀēČpĆÍ) midori@journeytoforever.org ÉWÉÉĀ[ÉjĀ[ĀEÉgÉDĀEÉtÉHĀ[ÉGÉoĀ[āūČěČáāĶāńā≠āĺā≥āĘĀI ć°Ć„ā∆āŗÉvÉćÉWÉFÉNÉgāūźiāŖāńāĘā≠āĹāŖā…ā≤éxČáāĘāĹāĺāĮā‹āĶāĹāÁćKāĘāŇā∑ĀBā†āŤā™ā∆ā§ā≤āīāĘā‹āĶāĹĀB |
źHó∆Ė‚ĎŤĀEźHóŅĖ‚ĎŤāŐÉyĀ[ÉW
Ā`āPāOāOČ≠źlē™āŐźHó∆āūā‹ā©ā»ā¶āťźĘäEāŇ
ā»āļāWČ≠āŐźlā™čQā¶āťāŐā©Ā`
ćžē®ā…āśāťźAē®ĖŻāŐéŻó ā∆ďŃí•
źAē®ĖŻāŐéŻó Źáā…ēņā◊āĹąÍóó
-- alphabetical order
Other oil crops
Oils and esters characteristics
Iodine Values
-- High Iodine Values
-- Talking about the weather
Quality standard for rapeseed oil fuel
Cetane Numbers
National standards for biodiesel
Fuel properties of fats and oils
Fuel properties of esters
źAē®ĖŻāŐéŻó
ÉoÉCÉIÉfÉBĀ[É[ÉčĒRóŅāŐź∂éYó āÕźAē®ĖŻāŐĖŮāWäĄ
íćą”: āĪāŐźĒílāÕćTā¶āŖā»źĄó ĀBéņćŘāŐéŻó āÕćÕĒ|ŹūĆŹā»ā«ā…āśāŤĎŚāęā»ēĚā™ā†āťĀB
| ćžē® | 1ÉwÉNÉ^Ā[Éčā†āĹāŤāŐĖŻéŻó (kg) | 1ÉwÉNÉ^Ā[Éčā†āĹāŤāŐĖŻéŻó ĀiÉäÉbÉ^Ā[Āj | 1ÉGĀ[ÉJĀ[ā†āĹāŤāŐĖŻéŻó ĀiÉ|ÉďÉhĀj | 1ÉGĀ[ÉJĀ[ā†āĹāŤāŐĖŻéŻó ĀiēńćĎÉKÉćÉďĀj |
|---|---|---|---|---|
| ÉgÉEÉāÉćÉRÉV | 145 | 172 | 129 | 18 |
| ÉJÉVÉÖĀ[ÉiÉbÉc | 148 | 176 | 132 | 19 |
| ÉIĀ[ÉcĀiÉJÉČÉXĒěĀj | 183 | 217 | 163 | 23 |
| ÉčÉsÉiÉX | 195 | 232 | 175 | 25 |
| ÉPÉiÉt | 230 | 273 | 205 | 29 |
| ÉLÉďÉZÉďÉJāŐä£ĎáČ‘ | 256 | 305 | 229 | 33 |
| Ė»Č‘ | 273 | 325 | 244 | 35 |
| ÉwÉďÉvĖÉ | 305 | 363 | 272 | 39 |
| ĎŚď§ | 375 | 446 | 335 | 48 |
| ÉRĀ[ÉqĀ[ď§ | 386 | 459 | 345 | 49 |
| ąüĖÉźm | 402 | 478 | 359 | 51 |
| ÉwĀ[É[ÉčÉiÉbÉc | 405 | 482 | 362 | 51 |
| ÉgÉEÉ_ÉCÉOÉT(euphorbia) | 440 | 524 | 393 | 56 |
| ÉJÉ{É`ÉÉāŐéŪ | 449 | 534 | 401 | 57 |
| ÉRÉäÉAÉďÉ_Ā[ | 450 | 536 | 402 | 57 |
| ÉJÉČÉVÉiāŐéŪ | 481 | 572 | 430 | 61 |
| camelinaĀiÉAÉ}ÉiÉYÉiĎģĀj | 490 | 583 | 438 | 62 |
| ÉSÉ} | 585 | 696 | 522 | 74 |
| ćgČ‘ | 655 | 779 | 585 | 83 |
| ēń | 696 | 828 | 622 | 88 |
| ĖŻčň | 790 | 940 | 705 | 100 |
| ÉqÉ}ÉŹÉä | 800 | 952 | 714 | 102 |
| ÉRÉRÉAĀiÉJÉJÉIĀj | 863 | 1026 | 771 | 110 |
| óéČ‘ź∂ | 890 | 1059 | 795 | 113 |
| ÉPÉV | 978 | 1163 | 873 | 124 |
| ćōéŪ | 1000 | 1190 | 893 | 127 |
| ÉIÉäĀ[Éu | 1019 | 1212 | 910 | 129 |
| ÉqÉ}āŐéŪ | 1188 | 1413 | 1061 | 151 |
| ÉyÉJÉďÉiÉbÉc | 1505 | 1791 | 1344 | 191 |
| ÉzÉzÉo | 1528 | 1818 | 1365 | 194 |
| éXĆŤĖŻčň(ÉTÉďÉSÉAÉuÉČÉMÉä) | 1590 | 1892 | 1420 | 202 |
| É}ÉJÉ_É~ÉAÉiÉbÉc | 1887 | 2246 | 1685 | 240 |
| ÉuÉČÉWÉčÉiÉbÉc | 2010 | 2392 | 1795 | 255 |
| ÉAÉ{ÉJÉh | 2217 | 2638 | 1980 | 282 |
| ÉRÉRÉiÉbÉc | 2260 | 2689 | 2018 | 287 |
| ÉMÉlÉAÉAÉuÉČÉĄÉVĀiÉpĀ[ÉÄĖŻĀj | 5000 | 5950 | 4465 | 635 |
ĖŻā™ćŐāÍāťĎľāŐćžē®
PurdueĎŚäwāŐźVćžē®ĀEźAē®ÉZÉďÉ^Ā[Āithe Center for New Crops & Plant ProductsĀjā…āśāťĀuNewCrop SearchEngineĀvĀBÉLĀ[ÉŹĀ[ÉhĀuoilĀvāŇĆüćűā∑āťā∆200ĆŹāŗāŐÉqÉbÉgā™ā©ā¶āŃāńā≠āťĀBĆüćűĆčČ āÕŹŕć◊ÉfĀ[É^ā…ÉäÉďÉNā≥āÍāńāĘāťĀB
http://www.hort.purdue.edu/newcrop/SearchEngine.html
ĀuPlants For A FutureĀiĖĘóąāŐāĹāŖāŐźAē®ĀjĀvÉfĀ[É^ÉxĀ[ÉXĆüćű
ópďrē ā…ĆüćűĀiSearch by UseĀjā©ĀAāŗāĶā≠āÕāĽāŐĎľāŐópďrĀiOther UseĀjā…ĀuoilĀvā∆ďŁóÕāĶāńĆüćűā∑āťā∆460ĆŹā™ÉqÉbÉgā∑āťĀBĆüćűĆčČ āÕŹŕć◊ÉfĀ[É^ā…ÉäÉďÉNā≥āÍāńāĘāťĀB.
http://www.ibiblio.org/pfaf/D_search.html
ĖŻāŐéŪóřā…āśāťĖŻéČā∆ÉGÉXÉeÉčāŐďŃí•
|
|
|||||
|
|
|
|
|
||
|
|
|
|
|||
| ćōéŪĖŻ(h. eruc.) |
5
|
0
|
-2
|
97 - 105
|
55
|
| ćōéŪĖŻ(i. eruc.) |
-5
|
-10
|
-12
|
110 - 115
|
58
|
| ÉqÉ}ÉŹÉäĖŻ |
-18
|
-12
|
-14
|
125 - 135
|
52
|
| ÉIÉäĀ[ÉuĖŻ |
-12
|
-6
|
-8
|
77 - 94
|
60
|
| ĎŚď§ĖŻ |
-12
|
-10
|
-12
|
125 - 140
|
53
|
| Ė»Č‘ĖŻ |
0
|
-5
|
-8
|
100 - 115
|
55
|
| ÉgÉEÉāÉćÉRÉVĖŻ |
-5
|
-10
|
-12
|
115 - 124
|
53
|
| ěĹéqĖŻ |
20 - 24
|
-9
|
-6
|
8 - 10
|
70
|
| ÉpĀ[ÉÄäjĖŻ |
20 - 26
|
-8
|
-8
|
12 - 18
|
70
|
| ÉpĀ[ÉÄĖŻ |
30 - 38
|
14
|
10
|
44 - 58
|
65
|
| ÉpĀ[ÉÄÉIÉĆÉCÉďĖŻ |
20 - 25
|
5
|
3
|
85 - 95
|
65
|
| ÉpĀ[ÉÄÉXÉeÉAÉäÉďĖŻ |
35 - 40
|
21
|
18
|
20 - 45
|
85
|
| čćéČĀiÉ^ÉćĀ[Āj |
35 - 40
|
16
|
12
|
50 - 60
|
75
|
| ÉČĀ[Éh |
32 - 36
|
14
|
10
|
60 - 70
|
65
|
ÉąÉEĎfČŅ
āśā§āĽĀ]ā©ĀyóÄĎfČŅĀz
ĖŻéČ100ÉOÉČÉÄā™čzéŻā∑āťóÄĎfāŐÉOÉČÉÄźĒĀBāĪāŐílā™ĎŚāęāĘéČĖbé_āÕĀAēsĖOėaéČĖbé_āūĎĹā≠ä‹ā›ĀAēsĖOėaďxā™ćāāĘĀBóÄĎfČŅ100ą»Čļāūēsä£źęĖŻĀA100Ā`130āūĒľä£źęĖŻĀA130ą»Ź„āūä£źęĖŻā∆āĘā§ĀićLéęČĎĎśāTĒŇāśāŤĀj
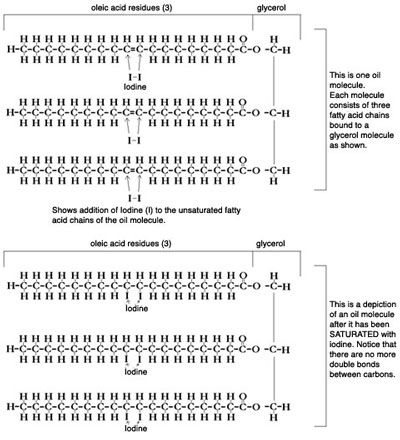
ČĽäwďIā…āĘā§ā∆ĀAā∑ā◊āńāŐźAē®źęĀEďģē®źęāŐĖŻéČāÕāRƬāŐéČĖbé_ā™ÉOÉäÉZÉäÉďā…ĆčćáāĶāĹÉgÉäÉOÉäÉZÉäÉhĀB
Chemically, vegetable and animal oils and fats are triglycerides, glycerol bound to three fatty acids. Animal tallow/lard is saturated, meaning that in the fatty acid portion, all the carbon atoms are bound to two hydrogen atoms, and there are no double bonds. This allows the chains of fatty acids to be straighter and more pliable so they harden at lower temperatures (that's why lard is a solid).
As you increase the number of double bonds in a fatty acid, you reduce that ability for oils to gain a conformation that would make them solid, so they remain liquid. To picture it, imagine that you put a bunch of strings in a line. Now tie knots in various places on the strings and see how they don't fit together tightly.
To test a vegetable oil to see how many double bonds it has (how unsaturated it is) iodine is introduced to the oil. The iodine will attach itself over a double bond to make a single bond where an iodine atom is now attached to each carbon atom in that double bond. Higher iodine numbers do not refer to the amount of iodine in the oil, but rather the amount of iodine needed to "saturate" the oil, or break all the double bonds. Oils for the most part contain only trace amounts of iodine naturally.
How does this translate to biodiesel? When the fatty acid chains are broken from the glycerol and then re-esterified to methyl or ethyl groups, those fatty acids still have their double bonds. That means that the more double bonds, the lower the cloud point because they resist solidifying at lower temperatures. So, for instance, if you use lard or tallow, the biodiesel will solidify at a higher temperature because the fat it was formed from also solidified at a higher temperature.
(Image and text compliments of Jeff Welter)
High Iodine Values
[The information below refers to straight vegetable oil fuel, but is also useful to show which oils are suitable for making biodiesel and which may not be suitable.]
-- From "Waste Vegetable Oil as a Diesel Replacement Fuel" by Phillip Calais, Environmental Science, Murdoch University, Perth, Australia, and A.R. (Tony) Clark, Western Australian Renewable Fuels Association Inc.
http://www.shortcircuit.com.au/warfa/paper/paper.htm
Many vegetable oils and some animal oils are 'drying' or 'semi-drying' and it is this which makes many oils such as linseed, tung and some fish oils suitable as the base of paints and other coatings. But it is also this property that further restricts their use as fuels.
Drying results from the double bonds (and sometimes triple bonds) in the unsaturated oil molecules being broken by atmospheric oxygen and being converted to peroxides. Cross-linking at this site can then occur and the oil irreversibly polymerises into a plastic-like solid.
In the high temperatures commonly found in internal combustion engines, the process is accelerated and the engine can quickly become gummed-up with the polymerised oil. With some oils, engine failure can occur in as little as 20 hours.
The traditional measure of the degree of bonds available for this process is given by the 'Iodine Value' (IV) and can be determined by adding iodine to the fat or oil. The amount of iodine in grams absorbed per 100 ml of oil is then the IV. The higher the IV, the more unsaturated (the greater the number of double bonds) the oil and the higher is the potential for the oil to polymerise.
While some oils have a low IV and are suitable for use as fuel without any further processing other than extraction and filtering, the majority of vegetable and animal oils have an IV which may cause problems if used as a neat fuel. Generally speaking, an IV of less than about 25 is required if the neat oil is to be used for long term applications in unmodified diesel engines and this limits the types of oil that can be used as fuel. The table below lists various oils and some of their properties.
The IV can be easily reduced by hydrogenation of the oil (reacting the oil with hydrogen), the hydrogen breaking the double bond and converting the fat or oil into a more saturated oil which reduces the tendency of the oil to polymerise. However this process also increases the melting point of the oil and turns the oil into margarine.
As can be seen from the table below, only coconut oil has an IV low enough to be used without any potential problems in an unmodified diesel engine. However, with a melting point of 25 deg C, the use of coconut oil in cooler areas would obviously lead to problems. With IVs of 25-50, the effects on engine life are also generally unaffected if a slightly more active maintenance schedule is maintained such as more frequent lubricating oil changes and exhaust system decoking. Triglycerides in the range of IV 50-100 may result in decreased engine life, and in particular to decreased fuel pump and injector life. However these must be balanced against greatly decreased fuel costs (if using cheap, surplus oil) and it may be found that even with increased maintenance costs this is economically viable.
| ĖŻā≤ā∆āŐóZČūČ∑ďxā∆ÉąÉEĎfČŅ | ||
|
|
|
|
| ěĹéqĖŻ |
|
|
| ÉpĀ[ÉÄäjĖŻ |
|
|
| órďųĖŻéČĀiÉ^ÉćĀ[Āj |
|
|
| čćéČĀiÉ^ÉćĀ[Āj |
|
|
| ÉpĀ[ÉÄĖŻ |
|
|
| ÉIÉäĀ[ÉuĖŻ |
|
|
| ā–ā‹āĶĖŻ |
|
|
| ÉsĀ[ÉiÉbÉcĖŻ |
|
|
| ćōéŪĖŻ |
|
|
| Ė»Č‘ĖŻ |
|
|
| ÉqÉ}ÉŹÉäĖŻ |
|
|
| ĎŚď§ĖŻ |
|
|
| čňĖŻ |
|
|
| ąüĖÉźmĖŻ |
|
|
| ąŮĖŻ |
|
|
From "Waste Vegetable Oil as a Diesel Replacement Fuel" by Phillip Calais, Environmental Science, Murdoch University, Perth, Australia, and A.R. (Tony) Clark, Western Australian Renewable Fuels Association Inc.
http://www.shortcircuit.com.au/warfa/paper/paper.htm
Note: More Iodine Values in charts below.
Talking about the weather
Generally, the higher an oil's Iodine Value, the lower the temperature at which it solidifies. Different terms are used for this -- melting point (MP), cloud point (CP), cold filter plugging point (CFPP), and pour point (PP). In practice they all mean about the same. It matters with both SVO systems using straight vegetable oil as fuel and to biodiesel, but more so to SVO systems.
As vegetable oils cool, wax crystals form, and the oil goes cloudy. The crystals can form a film on filters, blocking the flow of fuel. The temperature at which this occurs varies widely according to the oil type, from well below freezing point to well above freezing point.
It even varies for the same type of oil: new food-grade rapeseed or canola oil is usually "winterized" so that it doesn't cloud in the fridge and put people off. It will work nicely down to -10ľC, but once it emerges from the fryer, partly hydrogenated, degraded and probably containing some tallow from the food fried in it, it will only stay liquid and not plug filters down to freezing point or just above.
If you want to use an SVO system in a cold climate, you need a system configured to deal with the CFPP factor, and you need oil with a low CFPP. Coconut oil, palm oil, tallow and lard won't do, rapeseed or canola, soy, sunflower or corn oil are much better.
But if you live in a hot climate, cloud points won't bother you and the opposite is true: coconut and palm oil, tallow and lard all have higher cetane numbers than the others, and lower Iodine Values.
For biodiesel, the same applies, but to a lesser degree -- with most oils and fats, converting it into biodiesel tends to lower the CFPP. Biodiesel made with ethanol usually has a lower CFPP than biodiesel made with methanol. Additives and fuel-line heaters can solve the problem, and so can adding a proportion of petro-diesel or kerosene (up to 30% is usually recommended).
Quality Standard for Rapeseed Oil
| Quality Standard for Rapeseed Oil as a Fuel (RK-Qualitätsstandard) | ||||
|
|
|
|
|
|
|
|
|
|||
|
|
||||
| Density (15ľC) |
|
|
|
DIN EN ISO 3675 DIN EN ISO 12185 |
| Flash Point by P.-M. |
|
|
|
DIN EN 22719 |
| Calorific Value |
|
|
|
DIN 51900-3 |
| Kinematic Viscosity (40ľC) |
|
|
|
DIN EN ISO 3104 |
| Low Temperature Behaviour |
|
|
|
Rotational Viscometer (testing conditions will be developed) |
| Cetane Number |
|
|
|
Testing method will be reviewed |
| Carbon Residue |
|
|
|
DIN EN ISO 10370 |
| Iodine Number |
|
|
|
DIN 53241-1 |
| Sulphur Content |
|
|
|
ASTM D5453-93 |
|
|
||||
| Contamination |
|
|
|
DIN EN 12662 |
| Acid Value |
|
|
|
DIN EN ISO 660 |
| Oxidation Stability (110ľC) |
|
|
|
IS0 6886 |
| Phosphorus Content |
|
|
|
ASTM D3231-99 |
| Ash Content |
|
|
|
DIN EN ISO 6245 |
| Water Content |
|
|
|
pr EN ISO 12937 |
Abteilung Technologie nachwachsender Rohstoffe
Arbeitsgruppe Pflanzenöle
Department of Technology, regenerating raw materials
Working Group On Vegetable Oils
Dr. Bernhard Widmann
LTV-Work-Session on Decentral Vegetable Oil Production, Weihenstephan
http://dec2.tec.agrar.tu-muenchen.de/pflanzoel/rkstandard_e.html
| Comparison of properties of diesel, canola oil and commercial US biodiesel | |||
| . |
|
|
|
| Density kgL-1 @ 15.5 deg C |
|
|
|
| Calorific value MJL-1 |
|
|
|
| Viscosity mm2s-1 @ 20 deg C |
|
|
|
| Viscosity mm2s-1 @ 40 deg C |
|
|
|
| Viscosity mm2s-1 @ 70 deg C |
|
|
|
| Cetane number |
|
|
|
From "Waste Vegetable Oil as a Diesel Replacement Fuel" by Phillip Calais, Environmental Science, Murdoch University, Perth, Australia, and A.R. (Tony) Clark, Western Australian Renewable Fuels Association Inc.
http://www.shortcircuit.com.au/warfa/paper/paper.htm
1. Sims, R. Yields, Costs and Availability of Natural Oils/Fats as Diesel Fuel Substitutes, Report No LF2021 for the Liquid Fuels Trust Board, Wellington (NZ) 1982
2. Environment Australia (National Heritage Trust) (2000b). Setting National Fuel Quality Standards – Paper 2 - Proposed Standards for Fuel Parameters (Petrol and Diesel), Canberra
3. Beer, T., Grant, T., Brown, R., Edwards, J., Nelson, P., Watson, H., Williams, D. (2000) Life-Cycle Emission Analysis of Alternative Fuels for Heavy Vehicles. CSIRO, Australia
Cetane numbers
Cetane numbers rate the ignition properties of diesel fuels, just as octane numbers determine the quality and value of gasoline (petrol). It's a measure of a fuel's willingness to ignite when it gets compressed. The higher the cetane number, the more efficient the fuel. Biodiesel has a higher cetane number than petrodiesel because of its oxygen content.
From the Lubrizol Corporation:
http://www.lubrizol.com/ReadyReference/GasolineDieselFuels/default.htm
Ignition Quality or Cetane Number -- This factor influences ease of starting, duration of white smoking after start-up, drivability before warm-up and intensity of diesel knock at idle. Studies have correlated ignition quality with all regulated emissions. As ignition delay is reduced, the combustion process starts earlier and emissions (primarily carbon monoxide and hydrocarbons) are reduced.
Ignition delay is measured by the Cetane Number (CN) test (ASTM D 613), which uses a single-cylinder, variable compression ratio engine analogous to the Octane Number engine. In this case, the ignition delay of the test fuel is measured at a fixed compression ratio. This result is compared with the results from standard reference fuels consisting of blends of n-cetane and heptamethylnonane.
Diesel engines vary widely in their cetane requirements, and there is no commonly recognized way to measure this value. In general, the lower an engine's operating speed, the lower the CN of the fuel it can use. Large marine engines can tolerate fuels with CNs as low as 20, while some manufacturers of high-speed passenger car diesel engines specify 55 CN fuel.
National standards for biodiesel
| Comparison of different national standards for biodiesel | |||||||
| - |
|
|
|
|
|
|
|
| Standard / Specification |
|
|
|
|
|
|
|
| Date |
|
|
|
|
|
|
|
| Application |
|
|
|
|
|
|
|
| Density 15°C g/cm |
|
|
|
|
|
|
|
| Viscos. 40°C mm2/s |
|
|
|
|
|
|
|
| Distillat. 95% °C |
|
|
|
|
|
|
|
| Flashpoint °C |
|
|
|
|
|
|
|
| CFPP °C (cold filter plugging point) |
|
|
|
|
|
|
|
| Pour point °C |
|
|
|
|
<-15 |
|
|
| Sulfur % mass |
|
|
|
|
|
|
|
| CCR 100% % mass |
|
|
|
|
|
|
|
| 10% dist. resid. % mass |
|
|
|
|
|
|
|
| Sulfated ash % mass |
|
|
|
|
|
|
|
| (Oxid) Ash % mass |
|
|
|
|
|
|
|
| Water mg/kg |
|
|
|
|
|
|
|
| Total contam. mg/kg |
|
|
|
|
|
|
|
| Cu-Corros. 3h/50°C |
|
|
|
|
|
|
|
| Cetane No. |
|
|
|
|
|
|
|
| Neutral. No. mgKOH/g |
|
|
|
|
|
|
|
| Methanol % mass |
|
|
|
|
|
|
|
| Ester content % mass |
|
|
|
|
|
|
|
| Monoglyceride. % mass |
|
|
|
|
|
|
|
| Diglyceride % mass |
|
|
|
|
|
|
|
| Triglyceride % mass |
|
|
|
|
|
|
|
| Free glycerol % mass |
|
|
|
|
|
|
|
| Total glycerol % mass |
|
|
|
|
|
|
|
| Iodine No. |
|
|
|
|
|
|
|
| C18:3 and high. unsat.acids % mass |
|
|
|
|
|
|
|
| Phosphor mg/kg |
|
|
|
|
|
|
|
|
Alkalinity mg/kg
|
|
|
|
|
|
|
|
RME: Rapeseed oil methyl ester
FAME: Fatty acid methyl ester
VOME: Vegetable oil methyl ester
FAMAE: Fatty acid mono alkyl ester
-- From "Lifecycle analysis for alternative fuels", CSIRO Australia, 1998
http://www.dar.csiro.au/res/ggss/Life_Cycle_Analysis_for_Alternative_Fuels.htm
CEN Diesel Fuel Specification (EN 590:1993):
http://journeytoforever.org/energiaweb/en590en.htm
New US Standard:
The US ASTM PS121-99 was a provisional standard, now replaced by ASTM D-6751.
|
|
|
| Flash point (closed cup) |
|
| Water and sediment |
|
| Kinematic viscosity at 40°C |
|
| Sulfated ash |
|
| Sulfur |
|
| Cetane |
|
| Carbon residue |
|
| Total glycerine (free glycerine and unconverted glycerides combined) |
|
| Phosphorous content |
|
Fuel properties of fats and oils
| Fuel-related properties and iodine values of various fats and oils | |||||||
| Oil or Fat |
|
|
|
|
|
|
|
| Babassu |
|
|
|
|
|
|
|
| Castor |
|
|
|
|
|
|
|
| Coconut |
|
|
|
|
|
|
|
| Corn |
|
|
|
|
|
|
|
| Cottonseed |
|
|
|
|
|
|
|
| Crambe |
|
|
|
|
|
|
|
| Linseed |
|
|
|
|
|
|
|
| Olive |
|
|
|
|
|
|
|
| Palm |
|
|
|
|
|
|
|
| Peanut |
|
|
|
|
|
|
|
| Rapeseed |
|
|
|
|
|
|
|
| Safflower |
|
|
|
|
|
|
|
| High-oleic safflower |
|
|
|
|
|
|
|
| Sesame |
|
|
|
|
|
|
|
| Soybean |
|
|
|
|
|
|
|
| Sunflower |
|
|
|
|
|
|
|
| Tallow |
|
|
|
|
|
|
|
| No. 2 DF |
|
|
|
|
|
|
|
CN = cetane number; CP = cloud point, PP = pour point, FP = flash point.
Iodine values combined from Applewhite, T.H., in Kirk-Othmer, Encyclopedia of Chemical Technology; Third Ed.; John-Wiley & Sons: New York, NY, 1980, Vol. 9; pp. 795-811; and Gunstone, F.D.; Harwood, J.L.; Padley, F.B. Lipid Handbook; Second Ed.; Chapman & Hall: London, 1994.
Fuel properties from Goering, C.E.; Schwab, A.W.; Daugherty, M.J.; Pryde, E.H.; Heakin, A.J. Trans. ASAE 1982, 25, 1472-1477 & 1483.
All tallow values from Ali, Y.; Hanna, M.A.; Cuppett, S.L. J. Am. Oil Chem. Soc. 1995, 72, 1557-1564 (no CN given, calcd. cetane index 40.15).
(From: "Biodiesel: The Use of Vegetable Oils and Their Derivatives as Alternative Diesel Fuels", G. Knothe, R.O. Dunn, and M.O. Bagby, in Fuels and Chemicals from Biomass, Washington, D.C.: American Chemical Society. Download full-text article (MS Word, 337Kb):
http://www.biodiesel.org/reports/GEN-162.doc
Fuel properties of esters
| Fuel-related physical properties of esters of oils and fats | ||||||
| Ester |
|
(kJ/kg) |
(mm2/s) |
(deg C) |
(deg C) |
(deg C) |
|
|
||||||
| Cottonseed 2 |
|
|
|
|
|
|
| Rapeseed 3 |
|
|
|
|
|
|
| Safflower 4 |
|
|
|
|
|
|
| Soybean 5 |
|
|
|
|
|
|
| Sunflower 6 |
|
|
|
|
|
|
| Tallow 7 |
|
|
|
|
|
|
|
|
||||||
| Palm 8 |
|
|
|
|
|
|
| Soybean 5 |
|
|
|
|
|
|
| Tallow 9 |
|
|
|
|
|
|
CN = cetane number; CP = cloud point, PP = pour point, FP = flash point.
1. Some flash points are very low. These may be typographical errors in the references or the materials may have contained residual alcohols.
2. Geyer, S.M.; Jacobus, M.J.; Lestz, S.S. Trans. ASAE 1984, 27, 375-381.
3. Peterson, C.L.; Korus, R.A; Mora, P.G.; Madsen, J.P. Trans. ASAE, 1987, 30, 28-35.
4. Isiigür, A.; Karaosmanolu, F.; Aksoy, H.A.; Hamdallahpur, F.; Gülder, Ö.L. Appl. Biochem. Biotechnol. 1994, 45-46, 93-102.
5. Bagby, M.O. In Proc. 9th Int. Conf. Jojoba Uses, 3rd Int. Conf. New Industr. Crops Prod.; Princen, L.H., Rossi, C., Eds.; Assoc. Advancem. Industr. Crops. publ. 1996; pp. 220-224.
6. Kaufman, K.R.; Ziejewski, M. Trans. ASAE 1984, 27, 1626-1633.
7. Ali, Y.; Hanna, M.A.; Cuppett, S.L. J. Am. Oil Chem. Soc. 1995, 72, 1557-1564.
8. Avella, F.; Galtieri, A.; Fiumara, A. Riv. Combust. 1992, 46, 181-188.
9. Nelson, L.A.; Foglia, T.A.; Dunn, R.O.; Marmer, W.N. submitted for publication.
(From: "Biodiesel: The Use of Vegetable Oils and Their Derivatives as Alternative Diesel Fuels", G. Knothe, R.O. Dunn, and M.O. Bagby, in Fuels and Chemicals from Biomass, Washington, D.C.: American Chemical Society. Download full-text article (Acrobat file, 901 Kb):
http://www.biodiesel.org/resources/
reportsdatabase/reports/gen/gen-162.pdf
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Bubble washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?