Our definition of alcohol fuel is a nearly 100 percent alcohol with a tad of water in it -- not a blend of alcohol with gasoline. So ... why an alcohol fuel? And why not a blend of gasoline and alcohol.?
There are several reasons why we chose an alcohol fuel. The first and probably most important one is that alcohol can be made by anyone, with a minimum of equipment. The knowledge necessary to make it can be obtained just by reading this book. As long as folks can grow certain plants, they can make alcohol fuel to run all or part of their power equipment. Dependence upon someone else to supply that fuel is no longer a problem or a threat. Second, alcohol is a good fuel, superior to gasoline in many ways: It can give extra power to certain engines, it is almost non-polluting compared to gasoline, it is safe and easy to handle. Third, the cost of conversion from gasoline to alcohol is inexpensive: For many engines it is merely an adjustment of the carburetor jets.
Why not a gasohol fuel? The problem is water. Water and alcohol are totally miscible liquids. That is, they mix in all proportions. Pure alcohol and gasoline are also miscible liquids. But water and gasoline are not. This means that an alcohol-and-gasoline blend must be almost free of water. To make a 200-proof alcohol on the farm would require expensive equipment and additional production expenditures. At this time, that added expense would price a fuel blend beyond reason. But alcohol of 167 proof (16.5% water) is as good a fuel as 200-proof (100%) alcohol and better than gasohol.
Really, it comes down to basic survival. Right now, the fuel shortage doesn't seem all that serious. It's something like having a leaking roof: When it isn't raining, the problem is not so bad ... but when it is raining? The bottom line to all this is that when the next fuel shortage comes -- and you can bet that one will -- the ones who have prepared best will survive with the least pain.
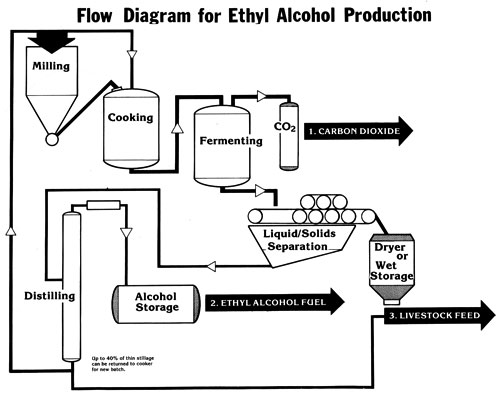
Introductory Overview of the Alcohol Production Flow Chart
The process of making alcohol fuel is not complicated. However, certain steps in the production line must be adhered to or else efficiency falls off drastically. The Ethyl Alcohol Production Flow Chart shows one system that works when using dry starch, such as that found in grain crops.
The first step is to mill the grain. There is no one essential machine for doing this. However, the particles of the grain must be small enough so that all the starch granules can be gelatinized in the cooker. If particles are too large, the starch granules will be too deeply embedded in the matrix of the seed to be gelatinized and therefore will not be converted to sugar.
Cooking dry starch in a water slurry is one of the best methods of preparing the granule for hydrolysis of the starch chain. Although some starch granules -- such as those in potatoes -- need not be boiled, the starch granule in corn is too hard for mere soaking of the grain to produce a high percentage of conversion. The cooker should have an agitator built into it to keep the starch chain in suspension in the liquid at all times. This insures even cooking and also prevents hot spots (which can scorch the solids on the bottom in a direct heat cooker).
After the boil, hydrolysis (the breaking down of the starches to sugars) occurs. Hydrolysis can take place in the cooker, and that will probably be the best arrangement for a small distillery.
Cooling after the cook is part of the hydrolytic process, and if the cooker has steam coils, these can be used as cooling coils as well (the same as with a steam-jacketed cooker). Adding extra water is another method of cooling (as explained in the section on Basic Steps in the Production of Ethyl Alcohol). The mash should be agitated during the cooling phase as well as the heating phase of mashing.
Following hydrolysis comes fermentation. Because yeast cannot tolerate large amounts of iron, a separate tank should be provided for this phase: The fermenting vat needs to be made of wood, stainless steel, or a coated mild steel. In some large whiskey distilleries, the vat is open to the atmosphere; this does not present any contamination problems, because the big commercial firms distill immediately after fermentation and practice good sanitation. If the farm distillery is not as clean, however, then the tank should be covered. In case of a totally enclosed vat, the access hole should be only large enough to allow a person to enter, and it must be covered when not in use. An airlock on the tank is not necessary, but do not allow any contaminants, such as dust or insects, to enter.
Somewhere along the production flow -- if a batch still is being used -- a decision will have to be made as to the point at which the solids will be separated from the liquid. The solids are likely to settle to the bottom and scorch. Separation can take place after hydrolysis or after fermentation. The machinery for this process on a small scale is almost nonexistent. Rotary screens, perforated tubing with augurs, and wringing out in a gunnysack are some of the methods being used, but you will probably have to find your own solution. With a continuous-feed perforated-plate column still, however, there is no problem. Distill the solids with the liquid mash, and feed the spent grains with the liquid (see the section on Distiller's Feeds)
Immediately after fermentation, distill! Each hour after the mash is ready, other bacteria will be working their way into the potential alcohol fuel. Acetic acid will not work too well in a car, and that is what the alcohol turns into when the Acetobacter bacteria invades the ferment.
As to the type of still to use, the choice depends upon the needs of the producer (see the section on Still Designs to determine which type of still will meet your own requirements).
Once the alcohol fuel is made, it will have to be stored. Use the same precaution that is used for gasoline storage. Alcohol is hygroscopic (absorbs moisture), so keep the vents in the storage tank small.
PRODUCTS FROM ETHYL ALCOHOL FERMENTATION
1. C02
One-half of the fermenting sugar is converted to carbon dioxide. It can be used for industrial application or in greenhouses for increasing plant growth.
2. ALCOHOL
The other half of the fermenting sugar is converted to ethyl alcohol. Since it contains all the fusel oil, esters, and aldehydes , it is not good for drinking, but a durn good fuel.
3. DISTILLER'S GRAIN
Nearly all the protein is left in the solids, so distiller's grain becomes a high-quality feed for livestock. Protein is 28-30% ; fiber is 12-13%; and moisture is 8-12%. Use it as a supplement to increase the protein in other feeds.
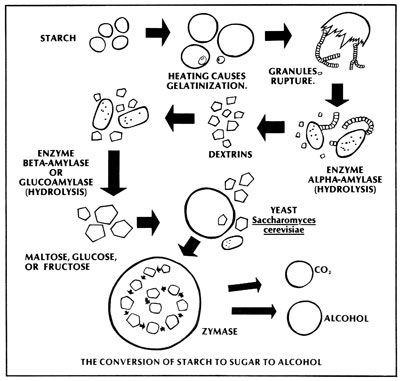
A Short But Complex Story About Enzymes and Their Functions
Just how important is it for you to understand the technical side of alcohol fuel production? After all, moonshiners -- for example -- have long made "white lightning" without knowing much about the inner workings of corn, sugar, and alcohol. On the other hand , the yield the oldtimers get from their raw materials is only a small fraction of the potential. So, if you're interested in getting the most from your time and effort, there's no substitute for knowing just what you're doing.
Ethyl alcohol -- the substance you're interested in making -- is an organic compound (C2H50H) which is also known as ethanol. Our alky closely resembles ethane (C2H6), one of the major by-products of gasoline refining. In fact, most of the commercially available ethanol in the U.S. is made from petroleum. However, sugar and starch crops are two other viable sources of alcohol.
The sugar extracted from cane or sweet sorghum can be directly fermented with little or no alteration, but the starches present in grains must be converted into sugars. Starch itself is nothing more than a long chain of individual glucose molecules, which must be broken apart or hydrolyzed with enzymes. However, the conversion process must be very carefully carried out, or your final alcohol yield will be seriously reduced.
The critters responsible for the transformation of starch to sugar and then sugar to alcohol are called enzymes, chemically known as protein biocatalysts. That hifalutin' word means that the enzymes are products of living cells, and that they encourage a chemical change without being consumed during the process. There are thousands of enzymes, and each one performs a specific task at an optimum temperature and acidity level (also known as pH). All enzyme names end in the suffix "ase", while the first portion of each of these terms describes the substance that enzyme specializes in converting. (For example, cellulase converts cellulose into sugar.)
All living cells produce enzymes, and grains are no exception. When a seed germinates, the enzymes are activated and begin the process of turning the stored food of the seed (starch) into a usable substance (sugar). Sprouted barley, for example, actually contains the right amount of enzymes to be used in an effective cooking process. Most grains, however, lack the proper amounts or kinds of enzymes to permit rapid, self-contained, complete conversion. Consequently, such grains need to have enzymes -- which are prepared from other sources -- added to the cooked meal during mashing.
It's important to understand that starch is actually a complex sugar. Each starch molecule is a long chain of up to a thousand glucose molecules bonded either in a straight line or branching like the leafless arms of a tree. Two enzymes are used to attack the "tree" at different points. Alpha enzyme attacks the branchpoints and reduces the tree into individual segments, while beta enzyme attacks the ends of each branch and nibbles off individual glucose molecules. In order to make all the starch available for enzyme activity, the carbohydrate granules must be held at a rapid rolling boil for 30 minutes. The heat causes the starch to expand and burst out of its cell wall, allowing our friends to get to work.
(Traditionally, barley malt has been used as an enzyme source, and also -- in the brewing industry -- as a flavoring agent. However, today's industrially prepared enzymes are more consistent and considerably less expensive.)
Mashing is basically a three-phase process which begins with the preboil. As the temperature approaches 150 deg F, the available starch begins to gelatinize, whereupon it is attacked by the alpha enzyme -- or alpha-amylase -- and reduced to a simpler carbohydrate. (The enzyme also serves to keep the mash from becoming too thick.) Subsequently, the mash is brought to a vigorous boil and held there for 30 minutes, to release all the remaining starch into solution.
In the postboil stage alpha-amylase is reintroduced -- since the enzyme is destroyed at 200 deg F and above -- to hydrolyze any remaining starches into simpler sugars called dextrins. Once the mash has cooled to 90 deg F, yeast is added to the mixture, along with beta-amylase. The beta enzyme operates at the same temperature as the yeast and breaks down the dextrins to glucose for the yeast to consume.
During fermentation, the yeast produces its own internal enzymes. In fact, there are 11 separate internal stages that the yeast goes through while "brewing". Yeast is a faculative organism, which means that once it has begun to consume sugar, it has a choice between two processes: to reproduce or to digest. If oxygen is present, the yeast will merrily bud itself ... but if oxygen is in short supply, the fungi will produce waste in the form of carbon dioxide and -- you guessed it -- alcohol. Therefore, it's best to agitate the fermenting mash for about ten minutes to encourage reproduction, and then cover it up and let it stand
Remember, you're dealing with a fairly sensitive biological process. So, follow all directions, be sure all supplies are kept in cool, airtight containers, and keep equipment clean. The numerous undesirable, microscopic sweet-tooths that can find their way into your mash will give you something, but it won't smell too good, and you won't be able to put it in your gas tank.
Mother Earth Alcohol Fuel
Chapter 1
Introduction to a Farmer's Fuel ... Alcohol
Introductory Overview of the Alcohol Production Flow Chart
A Short But Complex Story About Enzymes and Their Functions
Chapter 2
Farm Crops for Alcohol Fuel
Raw Materials
More on Raw Materials
Feedstock Handling and Storage
Chapter 3
Basic Steps in the Production of Ethyl Alcohol
More On Conversion and Fermentation
Fermentation Addendum
Alcohol Yield
Chapter 4
Control of Infection by Planned Sanitation in the Production of Fuel or Gasohol Alcohol
Chapter 5
MOTHER's Mash Recipes for Alcohol Production
Important! Read Before Making Mash
Preparing a Mash From Saccharide-rich Materials
A Handy Hydrometer Jacket
Chapter 6
Distiller's Feeds
By-product Utilization
Animal Feed By-product
More Information On By-product Utilization
Chapter 7
How the Distillation Process Works
Packed Column
Perforated Plate
Bubble Cap Plate
Solar Stills
The Reasoning Behind MOTHER's Still Design
Still Operation
Making Your First "Run"
"Economizing" Your Alcohol Production
Chapter 8
Six-Inch Column Still Plans
Three-Inch Column Still Plans
Bill of Materials
Chapter 9
Two Low-cost Backyard Stills
How To Adapt Your Automobile Engine For Ethyl Alcohol Use
Ron Novak's Do-It-Yourself Water Injection System
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
 Mother’s Alcohol Fuel Seminar
Mother’s Alcohol Fuel Seminar