Chapter Three
The Fungus-Roots of Trees
'By tying a Cord to the hind-leg of a Pig, and driving him before them ... observing where he stops and begins to root, ... they are sure to find a Trufle'
-- Ray, Creation, 11, 1691.
Let us now inquire what is actually known about this regular association of trees with toadstools, and explore the history of the subject in so far as this is on record.
For our first clue we must go back more than sixty years, and examine the activities of a German botanist who, somewhat unexpectedly, found himself occupied with an inquiry into the habits and behaviour of truffles. The truffle fungi live in woodland soils, forming their edible spore-bearing parts -- the truffles of commerce -- out of sight among the tree roots, particularly those of oak and beech. There are several different species of these fungi, all closely related and of similar habit. One of them, Tuber aestivum, produces truffles that used to be collected for market in parts of southern England; dark-coloured rounded bodies two to four inches in diameter, found chiefly among the roots of beeches growing in calcareous soils. Another species, the French truffle, familiar in pâté de foie gras, native in France and elsewhere in Europe, produces truffles highly esteemed for their delicate and delicious flavour, and is commonly found beneath evergreen oaks, sometimes under the ordinary oak.
The collection of truffles is troublesome owing to their underground habit, sometimes as much as a foot below the surface, and for this reason the supply available for marketing has always been relatively limited and the price correspondingly high. In certain districts where they are regularly collected, for example, in the Perigord region of France, small dogs are trained to recognize the characteristic odour and so locate the truffles by scratching away the soil above them, as pigs were once used in England for the same purpose. In order to secure increased supplies, various empirical methods, such as scattering the peelings of fruit bodies or transporting soil from a fertile locality, have at times been used, but at the period we are considering, more than sixty years ago, nothing was known with certainty about the manner and growth of the truffle fungi or the reasons for the constant association of their fruit bodies with roots of various trees.
About the year 1881, the German Government of the day, knowing these facts and desiring to increase the supply of truffles available for market, approached a well-known botanist, Professor A. B. Frank of Berlin, with a request for help. In order to learn something of their cultural requirements, it was suggested that he should organize a research into the causes responsible for the rather erratic distribution of truffles in nature, giving particular attention to their soil preferences and the constant association with roots of certain kinds of trees. Although Frank undertook this task and observations were begun on an extensive scale, no practical results in respect to truffles were ever achieved; the exact causes that limit and determine the distribution of these fungi in woodlands are still a matter for botanical speculation, although the facts set out in the remaining part of this chapter are certainly not irrelevant in this connection. Frank was an able botanist and his failure to solve this problem was doubtless indirectly due to the diversion of his attention from the matter immediately in hand to certain remarkable discoveries made at an early stage of the inquiry.
As soon as Frank and his students began to examine carefully the roots among which truffles commonly grow, they found an unexpected state of affairs in respect to the root-systems formed by the trees concerned, these differing remarkably in certain particulars from the descriptions given in the current botanical textbooks.
The underground parts of a tree consist of an extensive system of branched roots, many of which become relatively massive and of great length. While serving to anchor the tree firmly to the soil, these also give rise by extensive branching to great numbers of more finely branched rootlets -- the 'fibrous roots' of the gardener -- developed chiefly in the upper layers of the soil and functioning as the chief feeding roots or organs for absorbing water and soluble food materials from the soil. It was in respect to the character of this fibrous part of the root-system of trees that Frank made his great discovery.
In the oaks and beeches growing in woodland soils he quickly observed that the greater part of the fibrous root system was made up of branched structures, shorter and thicker than ordinary rootlets, varying in colour from creamy yellow to brown or almost black, occasionally pinkish or bright golden-yellow (Pl. 11).
The appearance of these rootlets invited investigation of their anatomical features. Study of their structure in the laboratory revealed a state of affairs hitherto unsuspected by botanists, and obviously of great significance in relation to the absorption of food materials from the soil and therefore to the nutrition of the trees.
Structurally, every such rootlet was found to consist of a young root enclosed within a sheath of fungus mycelium continuous over the whole surface including the tip. The colour and thickness of this envelope or mantle varied not only with age but also in the different kinds of rootlets, and were obviously related directly with the characters of the hyphae composing it. The actual surface of the root embedded in this fungus mantle showed of course no trace of the root-hairs described in text-books as the chief absorbing organs of trees and other land plants, and was indeed cut off completely from direct contact with the soil.
 Plate 11a. Fungus-roots of beech. Above: Part of the 'fibrous' root system of a beech tree; rather less than natural size. Almost all the rootlets shown in the photograph are mycorrhizas, the younger rather club-shaped from increasing diameter as they become mycorrhizal; the older branching to form little mycorrhizal systems consisting of several rootlets. Plate 11a. Fungus-roots of beech. Above: Part of the 'fibrous' root system of a beech tree; rather less than natural size. Almost all the rootlets shown in the photograph are mycorrhizas, the younger rather club-shaped from increasing diameter as they become mycorrhizal; the older branching to form little mycorrhizal systems consisting of several rootlets.
|
As seen in thin sections under the microscope, the fungus mantle appeared as a more or less compact structure formed by interwoven hyphae, the outer surface in some cases smooth, in others bearing outward-growing branches that penetrate the soil. From the inner side of the mantle emerged hyphae that grew inwards between the cortical cells of the root, penetrating the middle layer of their common walls, and forming a continuous network of mycelium extending often as far as the limits of the central core of conducting or vascular tissues. In general, the hyphal threads composing this intercellular network, the Hartig net, did not penetrate the living cells around them. These cells were of the usual type found in the cortex of young roots, differing only in their tendency to be of larger size than usual, and with certain features in their internal structure indicating special activities. They showed other structural features of a minor kind, for example, the presence of a layer of cells immediately inside the mantle conspicuous in sections of the roots because filled with brown tannin (Pl. 10). Some of these short roots were simple and unbranched; a majority formed crowded branch systems almost coralloid in appearance. They were best developed in woodland soils, the upper layers of which were often densely filled with them.

Plate 11b. A small part of the root system shown in Pl. 11a. Magnified about 8 diameters.
|
Pursuing his investigations, Frank found that many near relatives of beech and oak -- hornbeam, alder, hazel, and chestnut -- had root systems of a similar kind. Extending these observations to pine, spruce, and other conifers he found an identical state of things, the 'short roots' of pine being easily recognizable because they branched in a characteristic manner, forking repeatedly into two equal parts (Pl. 12, 13).
As a plant physiologist, Frank believed that he had made a discovery of great significance in plant nutrition. He noted especially the absence of root-hairs, often replaced in position and distribution by hyphae extending from the fungus mantle into the soil, and the absence of any evidence of parasitic attack upon the root-cells such as occurred in diseased roots attacked by parasitic fungi; also the absolute uniformity and stability of the structural features, so very different from what might be expected in rootlets subjected to casual attack by soil parasites.
Describing his observations in a series of papers that have become classical in botanical literature, he explained his interpretation of them and stated his conclusions in a paper published in 1885. These organs of forest trees, he announced, are not ordinary roots; they are special organs, now recognized as such for the first time; in structure, partly root partly fungus; functionally, of great importance to the trees because the mycelium of the mantle in direct contact with the soil not only absorbs water and soluble salts as do the root-hairs and younger parts of ordinary roots, but is continuous with hyphae of the same species, whether of the truffle or other fungi, living and growing in the soil outside the roots.

Plate 12. Fungus-roots of Scots Pine (Pinus silvestris). Note the characteristic forking of each into two rootlets of equal length which, by repeated branching of this kind, give rise to 'coralloid' clusters of mycorrhizas. Magnified about 2 diameters.
|
Frank justly claimed that this soil mycelium could tap supplies of food locked up in the complex and insoluble organic constituents of the soil humus and thus inaccessible to ordinary roots. It was also stressed by him that the root components of these specialized organs were cut off from direct contact with the soil by their enveloping sheath of mycelium, and that therefore all water and food substances in solution must pass through the hyphae composing the mantle, and then through those of the intercellular net before entering the living cells of the root. Moreover, since these peculiar rootlets made up the greater part of the root-system concerned mainly with the absorption of nutrients in solution from the soil, this mode of absorption must be a matter of fundamental importance in the nutrition of the trees.
These are special organs hitherto unrecognized by botanists, reiterated Frank; they must not be called roots but fungus-roots -- Pilzwurzeln. And that is how the rather unwieldy name they still bear originated: fungus-roots, the Mykorrhizen of Frank (Gk. myco, fungus; rhiza, a root); each, in English, a mycorrhiza.
In justice, it must be mentioned that the discovery of regular association of mycelium belonging to soil fungi with roots of the higher plants had already been made and announced in 1881 by a botanist named Kamienski. His observations were made on the Indian Pipe (Monotropa Hypopitys), one of the curious so-called saprophytic flowering plants destitute of chlorophyll, not uncommonly found growing in close contact with the tree roots in beech woods.
In the course of his studies on the Indian Pipe, Kamienski examined also the fine rootlets of beech growing in close proximity to the roots of this plant and commented on the fact that the former showed structural features very similar to those of the Indian Pipe in respect to fungus infection.
Frank's re-discovery of this as a regular phenomenon affecting many species of trees, and his claim that the roots concerned should be regarded as distinct and specialized organs marked by the new name bestowed upon them, with the rapid extension of his own observations and those of his students to groups of plants other than forest trees, challenged and held the attention of contemporary botanists and stimulated them to further work on the subject.
During the last twenty years of the nineteenth century, one after another, papers describing in great detail the mycorrhizas formed by orchids, heaths, and many other kinds of plants appeared in the botanical journals. Frank himself very soon recognized that fungus-roots were formed regularly by many kinds of land plants; also that their structural features might differ sharply from those observed in the very characteristic ectotrophic mycorrhizas he had recognized and named in forest trees.
His interpretation of the part played by the latter in the nutrition of the host trees provoked a storm of controversy, the last mutterings of which are still occasionally heard. In his view, the regular association of fungus mycelium with the living tissues of roots was highly beneficial to the parent trees, the root-fungi conveying to their hosts not only water and salts but also organic substances derived from the soil humus in solution. They may indeed be called 'the foster-mothers of the trees', he asserted. This interpretation of the facts was particularly unpalatable to members of a newly formed school of botany who were devoting attention to diseases of the higher plants caused by parasitic fungi. Many of these diseases are caused by attacks on the roots by soil fungi and the botanists making a special study of them -- the first exponents of the new science of plant pathology -- expressed the opinion that mycorrhizas in general were merely symptoms of disease caused in this way! They chose to ignore the absence of any evidence that the roots were damaged by the hyphae, and the still more striking fact that the healthier and more vigorous the trees, the more abundant and active was their equipment of fungus-roots. Although outside the scope of our present inquiry, these controversial aspects are briefly discussed in a later paragraph.
We must now inquire what is known about the identity of the fungi that form these constant and curious partnerships with plant roots -- the root-fungi or mycorrhizal fungi, as they are called?
For many years after Frank's pioneer work, the interest of botanists centred mainly on the distribution of the mycorrhizal habit among plants and the structural features of different kinds of mycorrhizas formed by them. By 1915, the fungi responsible for two remarkable associations, those formed by a majority of orchids, and by members of the heath family respectively, had been identified.
In both cases these associations are of a highly individual kind, the relations of the host plants with their fungus partners extending far beyond the regular formation of fungus-roots or mycorrhizas. It was not until the twenties of the present century that the identity of many of the fungi responsible for forming the mycorrhizas of forest trees as described by Frank forty years earlier was firmly established. At first, the attention of workers on the subject was naturally attracted by the prevalence of sporophores of certain soil fungi in woodlands and about trees. Among the commoner were those of species of Amanita, Boletus, Russula, Lactarius, and Tricholoma, certain species of these genera being noted as constantly associated with particular tree species: the Fly Agaric, Amanita muscaria, and nearly related species with pine, spruce, and birch; some species of Boletus with pine and spruce; other species with beech and oak. The toadstools of another species of the last-named genus, Boletus elegans, deep golden yellow in colour, are so commonly found in and about larch woods and under larch trees that this fungus was described by a Swedish observer as 'following the larch as the dolphin follows the ship' (Pl. 2, 4, 5).
There was, in short, a great deal of what is called in law strong presumptive evidence for believing that these common woodland toadstools with a number of others were sporophores of the soil fungi, active mycelium of which was associated with the fungus-roots of the various trees concerned. Theoretically, it might be thought easy to secure trustworthy evidence about this by tracing mycelium from the base of a sporophore to the surface of a young mycorrhiza. Indeed, observations of this kind resulted in the publication of long lists of woodland fungi claimed as the mycorrhiza-formers of oak, beech, and other tree species. Actually, as was soon realized by the more serious students of the subject, this method is not a safe and entirely satisfactory means under natural conditions for identifying the root fungi concerned. It would be possible, for instance, to observe mycelium extending from a particular toadstool to the surface of a mycorrhiza without being able to prove that this and no other is the mycelium responsible for forming the fungus-root. There are, indeed, only two ways by which conclusive proof about this can be secured, both laborious and involving the use of laboratory methods that test the knowledge and technical skill of the worker.
One method is to isolate the mycorrhiza-forming mycelium from a young fungus-root, and then grow it in 'pure culture', that is, free from any contaminating organisms, on suitable nutrients in the laboratory. The first of these operations is a difficult one because a root or mycorrhiza removed from the soil carries upon the surface particles of soil and many organisms, including bacteria and the mycelium and spores of fungi, and must therefore be subjected to a drastic cleansing and sterilizing procedure in order to remove these or inhibit their growth without impairing the vitality of the mycelium of the true root-fungus. If this preliminary sterilizing technique is successful, hyphae of the latter may be expected to grow out when the rootlet is 'planted' upon a suitable nutrient, thus forming a young colony of mycelium, portions of which may be transferred aseptically to other nutrients or treated in any way desired. These colonies must then be compared in detail with others formed by similar pure cultures isolated from sporophores of the suspected fungus.
The other method is even more difficult and laborious, although more satisfactory as a means of providing absolute proof. It involves growing pure cultures of both partners: the fungus to be tested and seedlings of the pine or other host tree, and then bringing them together in pure culture, that is, under conditions entirely free from contamination by any other organisms and otherwise under the control of the operator. By this means it may be determined whether under these conditions the mycelium concerned can form fungus-roots of the usual kind. A pure culture of the fungus to be tested is obtained by isolating mycelium from a sporophore as described above; pure culture seedlings can be grown by sterilizing the outsides of the seeds with a suitable antiseptic to remove surface contamination and then sowing them with aseptic precautions on a nutrient jelly in a flask or other suitable vessel,closed with a cotton-wool plug. Imperfect sterilization of the seeds is evidenced by growth of the contaminating organisms on the nutrient surface and seedlings from such seeds are rejected.
When large enough to handle easily, seedlings free from infection are then transferred to a vessel containing a suitable rooting medium, closed with a cotton-wool plug, and fitted with some arrangement for introducing fresh supplies of water and nutrients free from contamination as required. When the seedling begins to grow, mycelium from a pure culture of the fungus can be introduced with aseptic precautions.
If a suitable technique is used, and if such pure culture seedlings grow normally and produce rootlets that become mycorrhizas without signs of parasitism or of damage to the tissues by the attacking mycelium, such experiments afford final proof that the mycelium introduced is that of a fungus behaving as a mycorrhiza-former of the tree under natural conditions. In such experiments the difficulties presented by the provision of pure cultures of mycelium and seedlings are easily overcome; there is an established technique and only care and skill are required. Those presented by cultivation of the seedlings to a suitable age with the provision and maintenance of conditions favourable to the formation of mycorrhizas are much greater for the following reasons. The production of fungus-roots by a tree seedling growing under natural conditions is not a mere matter of propinquity: both young root and mycelium must be in a physiological state favourable to mutual interaction, this in turn being determined by conditions existing in the natural environment of the roots. The critical factors in this environment are at present incompletely known and must, therefore, be ascertained so far as possible and the requirements of both seedling and mycelium satisfied, especially in respect to aeration of the rooting medium and the supply of nutrients.
In spite of all these difficulties, the formation of mycorrhizas by a number of different fungi under such artificially controlled conditions has been observed, thus supplying final proof of the role played by their mycelium in woodland soils. By the application of these methods certain proof has been secured of the identity of many of the root-fungi of trees and strong indications of that of many others (Pl. 14).
Much of the knowledge so acquired is due to the skilful and laborious researches of Professor Melin of Upsala University, who has provided lists of woodland fungi that are proved mycorrhiza-formers of various trees growing in Swedish woodlands. Other workers elsewhere have added to these lists and there is now a considerable body of accurate information about the root-fungi of Scots pine, Mountain pine, and other species of pine, Norway spruce, larch, birch, etc., as well as much evidence just short of definite proof concerning the identity of many others. These fungi belong to many of the common toadstool-forming fungi of woodlands. Among the commoner and more important are species of Boletus, especially B. luteus, B. badius, B. variegatus, B. granulatus, and B. bovinus; the Fly Agaric, Amanita muscaria, also with great probability other species of the same genus mentioned in an earlier chapter; Lactarius deliciosus, producing a beautiful deep orange-coloured toadstool that exudes a bright reddish-yellow milky juice when broken, common in pine and other coniferous woods; species of Russula, also common in woodlands of both coniferous and deciduous trees.
Species of Tricholoma, Clitocybe, and other genera may likewise be included as the root-associates of many trees. Concerning fungi belonging to the Puff-ball group, the Gastromycetes, there is not quite so much certain knowledge, but certain of them, e.g. species of Scleroderma, are probably root associates of beech, possibly also of oak, and of other trees. For one member of this group there is experimental proof for this claim; Rhizopogon luteolus with its subterranean sporophores is a proved mycorrhiza-former for several species of pine, probably for many. The photograph reproduced in Plate 16 was made in Australia from an experimental culture of an introduced North American pine, Pinus taeda, and shows a large spherical sporophore or puff-ball of this fungus just below the surface of the soil in the experimental pot connected by strands of mycelium with the mycorrhizas of the young trees. For the truffle fungi, members of an entirely different subdivision of the higher fungi, the Ascomycetes, there is as yet no experimental evidence but it seems not unlikely that species of Tuber and related Ascomycetes may be included among the mycorrhiza-formers of trees.
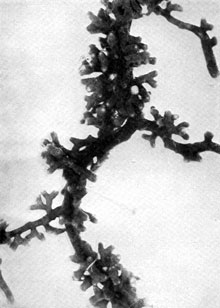 Plate 13. Fungus-roots of Scots Pine (Pinus silvestris). Some of the mycorrhizas shown in P1. 12 more highly magnified -- to about nine times (i.e. nine diameters) natural size. Plate 13. Fungus-roots of Scots Pine (Pinus silvestris). Some of the mycorrhizas shown in P1. 12 more highly magnified -- to about nine times (i.e. nine diameters) natural size.
|
Certain interesting facts emerge from even a cursory study of this very abbreviated list of common toadstools known to belong to the mycorrhizal fungi of various trees. One of these is the observation that a number of different fungi are mentioned as associates of the same tree species; another, that many of them apparently function as mycorrhiza-formers of different tree species. Both these deductions are correct. The trees that form the ectotrophic mycorrhizas we have been considering show a multiple habit in respect to their root-fungi, forming associations with a number of different woodland species, several of which may form different kinds of mycorrhizas simultaneously on different parts of the root. We may hazard a guess that this habit is perhaps not unconnected with soil or other differences related directly with the distribution of the fungi in nature, and also with the healthy growth of the trees. Moreover, as is apparent from consideration of even the small number of fungi mentioned here, many are mycorrhiza-formers for more than one kind of tree; for example, several of them are root-fungi of Scots pine and also of a number of other species of pine, as well as of Norway and other spruces. In this connection it is of interest to learn that many of these fungi have a very wide geographical distribution, toadstools of identical or closely related species of the same genera appearing commonly not only in British, Swedish, and other European woodlands, but also in those of North America, New Zealand, and South Africa.
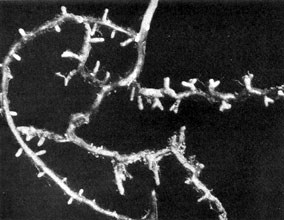
Plate 14a. Fungus-roots of White Pine (Pinus strobus), formed in a 'pure culture' of the seedling tree with mycelium of the Saffron Milk-cap, Lactarius deliciosus.
|
Many of the commoner trees in our British woodlands, with the conifers that compose the forests of Scandinavia and other countries of northern Europe and of North America, all produce fungus-roots of the type just described, ectotrophic mycorrhizas, to use the name given by Frank in order to distinguish them clearly from endotrophic mycorrhizas, in which mycelium of the fungus partner actually invades the living cells of the roots forming coils and branch systems of active hyphae within them. In external appearance mycorrhizas of the last-named type do not differ noticeably from ordinary roots. Internally, in spite of extensive infection by mycelium throughout the cortical tissues, they show no symptoms of suffering from parasitic attack, although the intracellular hyphae can, and probably do, take from the root-cells sugar and other organic food materials. This phase of fungus activity within the root comes to an end, however, with the onset of a digestive process on the part of the individual root-cells, resulting in the rapid disintegration of the mycelium and the gradual disappearance of most of the products of digestion from the root-cells. In this way food materials filched earlier from the host-plant are eventually returned in one form or another with, in addition, any others that may have reached the intracellular mycelium through hyphae connecting it directly with that growing in the soil outside the roots.
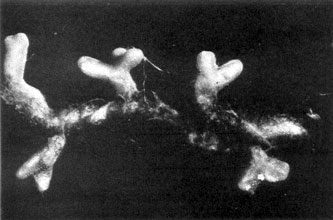 Plate 14b. Same as Pl. 14a, more highly magnified. Plate 14b. Same as Pl. 14a, more highly magnified.
|
As Frank and his fellow workers long ago discovered, the habit of forming root associations with mycelium of soil fungi is surprisingly widespread in both trees and herbaceous plants. In the latter, the mycorrhizas are always of the endotrophic type just described. This is so, for example, in orchids and in members of the heath family, two specialized groups from various members of which the root-fungi have been isolated and identified as belonging to soil fungi other than those of the toadstool-forming group.
In addition to such exceptional cases are great numbers of herbaceous species that form endotrophic mycorrhizas, among them many of the commoner crop plants of tropical and temperate regions, also many trees and shrubs. Among the latter are ash, walnut, sycamore, poplar, and members of certain families of conifers: the Monkey Puzzle (Araucaria spp.) and its relatives, the Umbrella Pine (Sciadopitys verticillata), the Big Trees and Redwoods of California (Sequoia gigantea and S. sempervirens) and members of the Cypress family, cypress, arborvitae and others. The buckthorn (Rhamnus cathartica) is a common British shrub possessing fungus-roots of similar kind.
The mycelium present in these endotrophic mycorrhizas, whether of trees or other kinds of plants, has many characters in common. It has resisted extraction from roots, and the fungi to which it belongs have therefore not been isolated or identified. They are believed to belong to a small but very widely distributed group of soil fungi, the affinities of which are still in doubt.
There is, it is true, a little evidence relating this kind of root infection in certain pasture grasses with the common mushroom. Apart from this, nothing is known to connect the mycelium responsible for it with the soil fungi of the toadstool-forming group. Further consideration of fungus-roots of this kind is therefore outside the scope of our present inquiry, beyond noting that it is already on record that certain trees and shrubs may form mycorrhizas of both kinds, for example, the Common Juniper (Juniperis communis) in Sweden. There is indeed some reason to believe that the distinction between the two kinds of structure marked by the names ectotrophic and endotrophic may really be less absolute than was believed in Frank's day, although the extreme types of structure are very distinct and easily recognized.
There is, therefore, a definite answer to our major problem touching the constant association of the toadstools of certain fungi with particular kinds of trees. Many of these toadstools are the sporophores of woodland fungi the mycelium of which has been proved experimentally to co-operate in the formation of the fungus-roots or mycorrhizas of the trees under which they grow; many others for which final proof is still lacking or has not been sought almost certainly play a similar role. Among the common genera of woodland fungi known to be mycorrhiza-formers for pine, spruce, larch, and birch are species of Boletus, Amanita, Lactarius, Russula, Cortinarius, and Tricholoma; there are probably others. The photograph reproduced in Plate 14 shows part of the root system of White pine (Pinus strobus), a common and widely distributed species of pine in the eastern United States, produced by the seedling pine growing in pure culture with mycelium of the mycorrhizal fungus, Lactarius deliciosus. Recent researches have confirmed the regularity of the appearance and constancy of structure of these tree mycorrhizas and made it certain that they play an important part in maintaining health and ensuring the proper nutrition of the host trees, especially in certain kinds of soils.,
These reciprocal relationships between trees and fungi must have had a long evolutionary history and it might be expected that among them will be found cases that provide indications of varying kinds and degrees of adjustment between the partners. There is evidence that some of the fungi concerned, for example, species of Boletus, cannot form their spore-bearing parts, and so complete their life history, except in association with their hosts. The actual nutritive relations that underlie these mycorrhizal partnerships are difficult to explore and still more difficult to prove, since it is clear that they are not only very complex but are also intimately bound up with the complicated systems that exist in natural soils and therefore difficult or impossible to reproduce in the laboratory for experimental purposes. Some of the possibilities are considered in the next chapter.
Next chapter
Back to the Small Farms Library Index

Community development | Rural development
City farms | Organic gardening | Composting | Small farms | Biofuel | Solar box cookers
Trees, soil and water | Seeds of the world | Appropriate technology | Project vehicles
Home | What people are saying about us | About Handmade Projects
Projects | Internet | Schools projects | Sitemap | Site Search | Donations | Contact us
|