Note
Making ethyl esters is a tricky process, not as simple as methyl esters. This paper provides the method, but you'll need to know some do's and don't's to make it work reliably. More information here.
See also Production and Testing of Ethyl and Methyl Esters
Transesterification Process to Manufacture Ethyl Ester of Rape Oil (Acrobat file, 672Kb)
Making and Testing a Biodiesel Fuel Made From Ethanol and Waste French-Fry Oil (Acrobat file, 2.5Mb)
Cornmeal Adsorber for Dehydrating Ethanol Vapors.
Anhydrous ethanol using sulphur or castor oil -- Separating Ethanol From Water Via Differential Solubility or Miscibility.
Absolute Alcohol Using Glycerine -- Mariller's absolute alcohol production process by dehydration using glycerine.
Optimization of a Batch Type Ethyl Ester Process
Authors: Charles Peterson, Gregory Möller, Randall Haws, Xiulin Zhang, Joseph Thompson and Daryl Reece
From: "Ethyl Ester Process Scale-up and Biodegradability of Biodiesel"
FINAL REPORT, No. 303, November 1996
For the United States Department of Agriculture, Cooperative State Research Service, Cooperative Agreement No. 93-COOP-1-8627
University of Idaho, College of Agriculture
Abstract
Conversion of rapeseed oil into ethyl esters for use as Biodiesel fuel involves transesterification of the oil triglycerides to mono-esters of the component fatty acids. To accomplish this conversion, raw rapeseed oil is treated at room temperature with ethyl alcohol in the presence of potassium hydroxide as a catalyst. During the process, the glycerol which is produced is insoluble in the ester product, and being heavier, settles out carrying most of the dissolved KOH catalyst with it.
Upon initial settling, some of the undesirable, emulsion-forming by-products may remain in the ester layer, causing problems in the washing stage. It was discovered (by tracking the process with a glycerol determination) that most of these products could be removed by simply restirring the glycerol into the ester, adding water and letting the mixture settle out again. After draining off the glycerol/water layer, the product (ethyl ester) can be easily water-washed to remove residual alcohol and potassium.
Introduction
Processing
Transesterification of rapeseed oil at the University of Idaho from 1980 to 1990 used methanol as the alcohol. Methanol is highly toxic, does not produce a visible flame when burning, can be absorbed through the skin, and is 100% miscible with water, so any kind of spill presents a serious problem. Ethanol provides the advantage of making a Biodiesel fuel produced entirely from renewable resources. The use of ethanol in Biodiesel production has not been studied as extensively as has methanol.
Water washing the ester was accomplished through sprinkling water into the tank at an approximate rate of 100 gallons per hour. As the water droplets travel through the ester, they remove the impurities. Washing would continue for 20 to 30 hours consuming as much as 3,000 gallons of water.
Oil Seed Press
Two commercially manufactured screw type oil expellers are used for extracting oil from the rapeseed for this project. The seed is manually fed into a 100 pound capacity tapered bin atop an auger. The seed is heated as it moves up the auger to the screw type press. A retention time of approximately 20 minutes is required for the seed to be heated before the oil is extracted. These two presses with augers were mounted on a movable base that were 12 feet in length, 4 feet wide, and 5 feet in height. Due to the size of these platforms and the limitations of shop space only one press could be feasibly operated at a time.
Reactor
A 290 gallon cross-link polyethylene reaction tank and a 50-gallon plastic rubbermaid barrel were used prior to this grant. The reactor tank was capable of producing 200 gallons of ester per week. A sink type drain was used with gaskets in the bottom of the reactor tank to drain the glycerol and then the wash water from the ester layer. The drain gasket was continually leaking and the cross-link polyethylene reaction tank was warping due to material compatibility with ester and glycerol. Fluids were pumped through the use of a centrifugal pump which required a hand primer pump to transfer the alcohol from the plastic barrel to the reactor tank.
Material and methods
Processing
Reactants
The reactants for the transesterification process are used in the following previously determined proportions in U.S. and metric units:
Raw rapeseed oil
100 L
100 Kg
100 Gal
Anhydrous Ethanol
27.4 L
23.74 Kg
27.4 Gal
Potassium Hydroxide
1.30 Kg
1.43 Kg
10.83 lb
The input amount of raw rapeseed oil determines the batch size, and the other components are calculated from the following formulas:
EtOH = 0.2738 x RO
KOH = 0.013 x RO
where:
EtOH = amount of ethanol required, in liters
RO = the desired amount of oil to be processed, in liters
KOH = amount of KOH required, in kg
According to these formulas ethanol is added at a 65% stoichiometric excess, or a molar ratio of 5.0: 1 EtOH to oil. The KOH is added at 1.43% of the weight of input oil.
Quality of Reactants
1. Rapeseed Oil. Best when clear (filtered) because excess sediment collects on the bottom of the reaction vessel during glycerol settling and at the liquid interface during washing. This sediment interferes with the separation of liquid phases and with the washout of catalyst, and may tend to promote stable emulsion formation. Slight haziness of the oil probably does no harm. The original oil must be water-free, because every molecule of water destroys a molecule of catalyst, thus decreasing its concentration.
2. Ethanol. The nearer to absolute (200 proof), the better. Gasoline present in the alcohol as a denaturant appears to do no harm. The reaction proceeds satisfactorily in mixtures of 200-proof ethanol with 10%(v/v) or more gasoline present. However, even small quantities of water (less than 1%) can decrease the extent of the conversion reaction enough to prevent the separation of glycerol from the reaction mixture.
3. Potassium Hydroxide Catalyst. Best if it has > 85% KOH. Even the best grades of KOH have 14 to 15% water (which cannot be removed). It should be low in carbonate, because potassium carbonate does not serve as a satisfactory catalyst, and may cause cloudiness in the final ester.
Other catalysts which may be used are potassium ethoxide and sodium ethoxide, but they are prohibitively expensive. Sodium hydroxide was not a suitable catalyst because it was not sufficiently soluble in ethanol and it tends to promote undesirable gel and emulsion formation during transesterification.
The Reactions
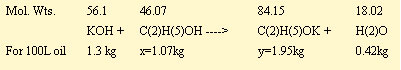
Using a 100 liter batch of oil as an example, the KOH used reacts with 1.07kg of ethanol to produce 1.95kg of potassium ethoxide. This mixture now contains (27.4x0.789)-1.07 = 20.55kg of free ethanol and 1.07kg of ethanol as potassium ethoxide catalyst. Any water added to the entire system reverses the above reaction and quenches a proportional amount of the potassium ethoxide catalyst. One part of water can quench up to 84.15/18.02 = 4.67 parts of catalyst.
The ethanol-KOH mixture is then poured into the rapeseed oil, and the following transesterification reaction occurs: (a hypothetical formula for the rapeseed oil, based on a typical oil analysis is used):

From these relationships, 100 liters (91kg) of rapeseed oil reacts with 13.1kg of ethanol. The 21.62kg (or27.4L) of ethanol used in the batch represents 21.62/13.1x100 = 165% of that required for complete transesterification of 100 liters of rapeseed oil. (A 65% excess over the theoretical requirement).
The Mechanics of the Transesterification Process
1. Raw rapeseed oil is measured into the reactor.
2. The required amount of ethanol is placed into a smaller covered container.
3. The required amount of potassium hydroxide is quickly weighed, protecting it as much as possible from atmospheric moisture and carbon dioxide.
4. The solid potassium hydroxide is added to all of the ethanol which is then vigorously stirred in the covered container until completely dissolved. At this point the dissolved KOH is presumed to have been converted to potassium ethoxide catalyst. Any undissolved pellets of KOH left in this alcohol tend to remain undissolved during the entire subsequent transesterification, essentially decreasing the amount of catalyst taking part in the reaction.
5. The ethanol-catalyst mixture is poured into the oil in the main reactor and stirred rapidly. Mixing is continued for 6 hours at room temperature. The reaction mixture usually changes to a turbid orange-brown color within the first few minutes; then it changes to a clear transparent brown color; finally, as the reaction is completed, the mixture again becomes somewhat turbid and orange-brown colored due to the emulsified free glycerol which has been formed.
6. In a good completed reaction, the glycerol begins to separate immediately upon cessation of stirring, and the settling mostly complete in one hour. After initial settling, the entire contents of the reaction vessel are again mixed together and stirred vigorously for 40 minutes. After the first 20 minutes of restirring, water is added at 15% of the initial volume of oil used in the reaction. Stirring should continue an additional 20 minutes after the water is added for a total of 40 minutes of restirring. This mixture is then allowed to settle overnight or over a weekend. A longer separation time facilitates the washing process. Remixing the glycerol layer with the ester layer while adding water has the effect of collecting and removing impurities and products of incomplete reaction from the ester. The washing phase can then proceed at a more rapid pace than if the remixing stage were left out.
In batches where poorer quality (moisture-containing) ethanol is used the reaction will not go to completion and requires much longer for the glycerol to separate. If separation does not occur, the addition of a small amount (perhaps 10% of the original volume) of alcoholic KOH with stirring may tip the reaction balance in favor of separation. It is also sometimes possible with the addition of a small amount of water (0.5% of the total volume) after the reaction is supposedly completed, to effect the separation of the glycerol from the ester. If the original ethanol contains as much as 1% water, the reaction may be so incomplete that the glycerol may never separate, and the entire batch must be discarded.
7. After remixing the glycerol and 15% water addition, and completion of the separation, the lower, heavier glycerol/water layer is drained off, pumped into barrels and shipped to a recycler. A thin layer of gross glycerol and sludge may adhere to the bottom of the reactor. It is advisable to wash down the cone of the reactor to remove this adhering glycerol or sediment by pouring a few gallons of cold water down and around the inside circumference of the reactor. This should be done at least twice. Any sediment probably consists of a host of minor components of the original rapeseed oil (proteins, glycoproteins, waxes, sterols, carotenoids, phosphatides, carbohydrates, etc.). Some of these constituents are emulsifying agents, and others have affinity for water which causes hold-up of undesirable impurities(e.g., potassium) and tend to prolong the washing process.
8. Finally, in order to remove the remaining alcohol and trace amounts of potassium, glycerol or soap, the ester is washed with water at about 30% of the ester volume or 30 gallons of water to a 100 gallon batch of ester. The water is stirred into the ester with mechanical stirring and air agitation as described in the next section. After a few hours the stirring/aeration is stopped and the water is allowed to settle out for two to three days. At this point the process is complete and the crystal clear product can be pumped into fuel tanks for storage or immediate use.
The Washing Process
Washing the ester product is necessary in order to improve its fuel properties, largely by removing residual free glycerol and small amounts of potassium remaining from the catalyst. The best method so far devised was previously described. It is a combination of: 1. Mixing the glycerol layer into the ester after the initial settling has occurred; adding 15% water; stirring and settling. 2. A water wash with agitation and aeration after the glycerol/water layer has been drained off.
Soap Formation
Soaps, at least in trace amounts, can be formed by an accompanying reaction during or subsequent to the transesterification process:
O O
RC-OC(2)H(5) + H(2)O-------- RC-OH + C(2)H(5)OH
Ethyl Ester Water Fatty Acid Ethanol
This is an equilibrium reaction, and any base will neutralize the acid formed, removing it, and forcing the reaction to the right. Also the reaction product of the base and acid is an undesired substance (a soap, which is an emulsifying agent).
O O
RC-OH + KOH-------- RC-OK + H(2)O
Fatty Acid A Base Salt/Soap Water
These reactions have little tendency to occur during the transesterification because of the small amount of water in the system. The source of the interfering water for this reaction may be use of low-grade water-containing ethanol, water in the other reactants at the beginning (from atmospheric exposure), or even from the first stage of the water wash. In any case, only a trace of soap needs be formed to promote emulsification of the ester with the wash water.
Wash Methodology
1. Agitation. During washing, many of the impurities in the ester have a greater affinity for the water, and they are transferred by diffusion across the phase boundary into the water. This process is greatly hastened by agitation, which can increase the area of phase contact by emulsion formation, or can promote transfer by maintaining the most effective concentration gradient for transfer across the interface.
2. Mechanical Stirring. Best results have been obtained using a mechanical stirrer whose rotation can be strictly controlled. The best speed for the equipment used has been about 50 to 70 RPM. The stirrer shaft should have two blades with one in the water phase and one in the ester phase rotating to lift the solution upward. This orientation, along with aeration develops maximum contact between ester and water.
3. Aeration Mixing. A unique method of aeration mixing was discovered. If air is introduced deep into the water layer through a sandstone, glass or stainless steel gas diffusion disk numerous air bubbles are formed in the water phase. These numerous water coated bubbles rise through the liquid interface into the ester, carrying large amounts of water in the film, and accomplishing washing as they rise up through the ester. Upon reaching the surface, the bubbles burst and form droplets of water which fall back down through the ester, further washing it. These bubbles and droplets seem to be of such size and nature that the droplets formed do not remain emulsified when they reach the aqueous phase, but quickly coalesce and disappear into the aqueous layer. This method greatly magnifies the interface area, and at the proper aeration rate, half or more of the ester phase volume seems to be filled with quite rapidly settling droplets of aqueous phase.
4. Combination Aeration and Mechanical Stirring. By combining (2) and (3) above very efficient and rapid washing can be achieved using minimum amounts of water.
Product Completeness
1. Bench Wash Test. Throughout the washing, a rough idea of the completeness of the washing may be obtained by washing (in a 100 ml beaker with magnetic stirring) 50 ml ester with 25 ml water for about 1/2 hour, and then determining the pH of the wash water. If the ester is sufficiently washed, the pH should be around pH 6 to 7. There is also a good way to determine washing completeness by noting the emulsion-forming tendency during this beaker wash-test. If the wash has been satisfactory, it is possible to stir a sample rather vigorously in this test and form an emulsion of large, clear, shiny droplets which will quickly separate, settle, and disappear upon cessation of stirring.
2. Turbidity. Occasionally a batch of washed ester may end up with turbidity caused by traces of condensed moisture. This moisture may be conveniently removed (evaporated) by aeration with dry air, using the gas diffusion disks from the washing step, to increase the surface contact between the air and the ester. Slight warming along with the aeration also hastens the removal of this trace of moisture.
Process Optimization
In the development of the above procedure for producing high quality ethyl esters, A.O.C.S. method Ca 14-56 for determining total, free and combined glycerol was used to follow the process. Procedural methodology was determined on the basis of percent glycerol left in the product after each step. Due to health and safety concerns, heptane was used as the solvent in place of the recommended chloroform. It was found to be very satisfactory. (n-Hexane and 2,2,4Trimethylpentane were not satisfactory)
Five sets of tests were made, each with 6 small beaker batches of REE. Batches were made according to the recipe previously described and allowed to settle for 30 minutes (except for the first one) before further treatment took place. Methods and inputs were generally kept constant except for the one being studied. Glycerol determinations were made on samples settled for at least 24 hours after treatment.
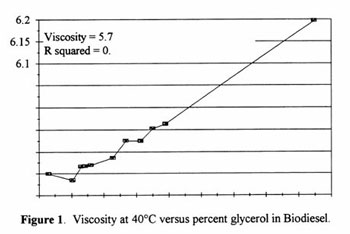
Viscosity vs. Total Glycerol
At first, a direct correlation between viscosity and glycerol was determined using A.O.C.S. Ca 14-15 (Figure 1). It was an effort to test the method for accuracy and reliability by spiking some relatively pure REE with various amounts of pure glycerol. The cluster of points at the lower end of the graph represent production grade product. It was found that this correlation only holds true for washed esters. REE at intermediate stages may contain some alcohol which affects the viscosity reading.
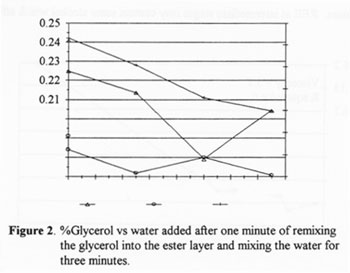
Addition of Water
1. Amount of Water. In this set all samples were allowed to settle then remixed with glycerol for one minute before water was added with the exception of the first one in which water was added at the end of the 2 hour reaction time. All samples were stirred for 3 minutes after water was added. At this stage of the process it was found that increasing water could be tolerated to the point where a permanent emulsion was created, which was approximately 30% by volume of the original amount of input oil. Figure 2 shows that water has very little effect on combined glycerol but that it significantly reduces free glycerol in the ester. Notice, however, that the free glycerol was plotted against the 2nd Y axis and that the numbers are quite small. The amount of water added to the mixture that effected the highest glycerol removal was found to be 20%. This figure will vary according to the quality of the raw products used in the reaction. Poorer quality reagents will produce a product higher in mono, di, and triglycerides and thus will tolerate less water before a permanent emulsion is formed. This was the reason that 15% water was recommended. Notice also in Figure 3 that a point in the upper left hand comer shows the effect of adding water at the end of the reaction without first letting the glycerol settle out. It was higher in glycerol, which suggests that the action of settling and remixing before adding water decreases combined glycerol. It can be said that some amount of mono, di and/or triglycerides are removed or converted by the glycerol only remix.
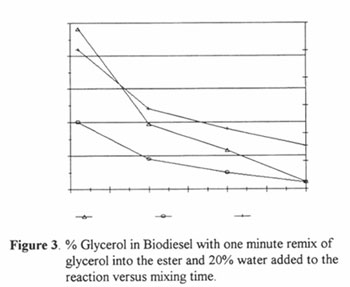
2. Time of Water Remix. In this set the small beaker batches were remixed with glycerol for one minute before the addition of water except for the first one which was stirred for 10 minutes before water was added. Water was added at 20% of the total for each sample. Stirring time varied from 1 to 30 minutes. In Figure 3 it can be seen that the glycerol decreased steadily with time. Twenty minutes was chosen as the cutoff time for the remixing. Data from the first point (10 min premix) confirmed the idea that combined glycerol can be reduced and prompted the next set of tests in which the glycerol only remix was lengthened.
Glycerol Remix
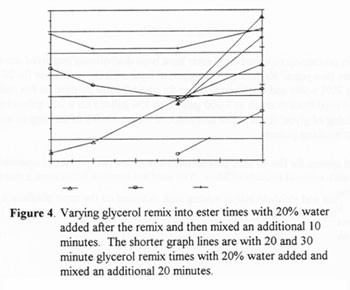
In this set of tests the glycerol only remixing time was varied from 5 to 30 minutes. All samples then had 20% water added and continued mixing for 10 min (first four) or 20 minutes (last two). Figure 4 shows that the combined glycerol does in fact decrease, confirming again that some amount of mono, di and/or triglycerides are removed or converted in this process. The free or dissolved glycerol actually increased with time, however the final water wash should effectively remove all but a trace of it. The lowest glycerol readings were from the sample which was restirred for a total of 40 minutes, 20 minutes in glycerol only mode and 20 minutes after the 20% water was added. These were the parameters chosen as a pretreatment to the final water wash and can be referred to the 20/20/20 rule for treating batch processed Biodiesel. As was stated earlier, the amount of water added should be reduced to 15% if the reagents are not of the highest quality.
Oil Seed Press
A seed press stand was designed and built for the seed to be above the press and gravity fed. This eliminated the need for an auger and reduced the size of the base to seven feet long, five feet wide, and a height of 11 feet. The seed bin was sized for a 2,000 pound capacity of seed and placed on a digital readout scale. The meal bin was positioned beneath the seed bin and was also placed on a digital readout scale for monitoring the press efficiency. A seed heating chamber was sized for the seed to have a retention time of twenty minutes before entering the screw-type press. The second press was positioned so the gravity feed seed tube was connected to the bottom of the existing funnel-type seed bin. This allowed for simultaneous press operation and a minimal amount of monitoring the presses and seed bins (due to the seed bin capacity of two days).
Reactor
A 400 gallon capacity 316 stainless steel, 10 gage cone bottom, open top tank was mounted on a 6 foot by 8 foot portable platform. An 80 gallon poly tank with a cone bottom for the alcohol and catalyst mixing was mounted next to the stainless steel reaction tank. The centrifugal pump was mounted below the plastic alcohol tank eliminating the need for a hand primer pump. An air driven transfer pump is used to transfer alcohol form barrels to the poly alcohol/catalyst tank. A hydraulic motor with a shaft and mixing blades was mounted to the top center of the reaction tank for the washing process. Aeration tubes for the washing process are introduced to the reaction tank during the washing process.
Conclusions
Developments in processing rapeseed ethyl ester have been dramatically improved during the course of the past two years. Remixing of the glycerol layer with the ester layer for 20 minutes and then adding 20% water and continuing mixing for an additional 20 minutes has reduced the amount of water used from as much as 3,000 gallons to 150 gallons for a 200 gallon batch of ester. The remixing of glycerol and water also reduced the possibility of forming an emulsion during the water washing process.
The gravity feed system for the oil seed presses has allowed for two presses to operate simultaneously with minimal amount of labor. The seed bin requires filling once a day and the meal bins are emptied every other day.
A larger reactor tank and alcohol/catalyst mixing tank mounted on the same platform has increased production by 1.3 times that of the previous reaction tank. Pumping procedures have been simplified along with handling of the alcohol.
Copies of the full report can be obtained from the Department of Biological and Agricultural Engineering, University of Idaho, Moscow, Idaho 83844-2060. The price charged will be that in effect at time of the order but not less than $100.00 U.S. plus shipping
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
