Chapter 1
AN OVERVIEW
ALCOHOL FUEL
There is nothing new in the use of alcohol as a motor fuel. In 1872, when Nikolaus Otto invented the internal combustion engine, gasoline was not available. Ethyl alcohol at 180-190 proof was the specified fuel. The model "T" Ford was designed to run on the available crude gasolines, alcohol, or any combination of the two.
Alcohols in general and ethanol, in particular, make excellent motor fuels. The reason alcohol fuel has not been fully exploited is that, up until now, gasoline has been cheap, available, and easy to produce. However, crude oil is getting scarce, and the historic price differential between alcohol and gasoline is getting narrower.
Currently there is a big push to find and develop alternative sources of energy so that dwindling reserves of crude oil and other fossil fuels may be conserved. As Edward Teller, one of this country's leading physicists points out: "No single prescription exists for a solution to the energy problem. Energy conservation is not enough. Petroleum is not enough. Coal is not enough. Nuclear energy is not enough. Solar and geothermal energy are not enough. New ideas and developments will not be enough by themselves. Only the proper combination of all of these will suffice."
Alcohol fuel can be an important part of the solution, but it is by no means a panacea. If all of the available agricultural surplus were converted to ethanol, alcohol would supply less than 5% of our motor fuel needs. Add the possibility of converting cellulose residues to ethanol and general biomass to methanol, and the most optimistic total falls short of 10% of our present needs! However, this is a very important 5 or 10% because it can be renewed each year, and each gallon of alcohol produced will save a gallon of oil.
USES OF ALCOHOL FUEL
One very important fact about alcohol fuel should be stressed. Alcohol is an excellent alternative motor fuel for gasoline engines. It is not a suitable alternative for home heating or for essentially stationary power requirements. The production of alcohol consumes energy. Exactly how much depends on the feedstock (raw material) and the efficiency of the distillation process. In a small operation, it would not be uncommon to expend 30-40,000 Btu per gallon of ethanol. It would be more sensible, in a home heating situation, to use whatever fuel you would use to run the still directly rather than using it to produce alcohol. The real advantage of alcohol is that it can be burned in the millions of existing vehicles with little or no modification. Alcohol fuel should only be considered for the jobs it can do best.
OTHER ALTERNATIVE FUELS
This book is about the small scale production of ethanol for use as a motor fuel. However, before becoming committed to ethanol, there are other alternatives that should be considered.
The first that comes to mind is methanol, or "wood" alcohol. Like ethanol, methanol is a viable substitute for gasoline, and it can be produced from a wide variety of renewable biological resources. Methanol, however, is not as easy to produce on a small scale.
The simplest and oldest method of producing methanol is by the destructive distillation (pyrolysis) of wood. The process is nothing more than heating the wood residues in a "dry" distillation apparatus and collecting the methanol at the other end. As such, the process requires relatively simple equipment and should be suitable for small scale production. The problem, then, is the fact that along with the methanol a considerable amount of impurities are produced that include acetone, acetic acid, and a number of other substances. These by-products are difficult and expensive to remove, and, if left in the methanol, they will quickly corrode an engine. Simply put, the small scale production of methanol by destructive distillation requires a large enough plant to justify the equipment and energy necessary to remove the impurities. If you happen to have a large source of suitable hardwood and are prepared to make the necessary capital investment, methanol production by this method might be considered.
Other processes for producing methanol from renewable resources, such as hydrogen and carbon monoxide, or conversion of cellulose and biomass, also exist. Again, the problem is that these methods are only feasible on a very large scale.
Methane gas has also been considered as a motor fuel. Methane is generated, for example, by the action of bacteria on manure. The problem here is that any methane production facility must be large enough to justify the equipment and energy required to compress the gas for storage. Also, methane has a very low heat value (energy content per unit of weight) and engine conversion is necessary. Methane is better suited to stationary power requirements than for use as a motor fuel. Still, if you have a situation where a large amount of manure or other suitable biomass is available, methane generation should be considered.
Natural gas, propane, and butane are also possible motor fuels. However, since all of them are basically petroleum related, they cannot be considered as renewable resources.
Much research has been done to find better processes for separating water into hydrogen and oxygen in order to obtain the hydrogen for use as a fuel. To date no process has been developed that does not consume more energy than can be returned when the hydrogen is burned.
Aside from alcohol and, perhaps, methane, there seems to be no other suitable alternative fuel that can be made from renewable resources and utilized in existing motor vehicles. Other means of powering vehicles, such as electricity, involve the development and production of completely new vehicles. What seems to be needed is a vehicle that can utilize a wide variety of fuels such as coal, wood, alcohol, gasoline, kerosene, corn cobs or whatever might be available--for instance, something similar, to the 1897 Stanley Steamer!
But, in the meantime, ethanol is the best solution for a motor fuel from renewable resources that can be produced easily on a small scale.
Chapter 2
BASIC FUEL THEORY
CHEMICAL COMPOSITION
Alcohol and gasoline, despite the fact that they are from different chemical classes, are remarkably similar. Gasoline is mostly a mixture of "hydrocarbons". Hydrocarbons are a group of chemical substances composed exclusively of carbon and hydrogen atoms. This is a very large chemical class containing many thousands of substances. Most of the fuels we use such as coal, gasoline, kerosene, fuel oil, butane, propane, etc. are chiefly hydrocarbons. Referring to Figure 2-1, the simplest member of this group is methane which consists of a single carbon atom and four hydrogen atoms. Next comes ethane with two carbons and six hydrogens. Propane has three carbons and butane has four. The substances just named are gases under ordinary conditions. As we add more carbons to the hydrocarbon molecule, the chemicals formed become liquids: pentane, hexane, heptane, octane and so on. As we continue with even more complex molecules, the substances get progressively oilier, waxier and finally solid.
Figure 2-1: CHEMICAL STRUCTURES
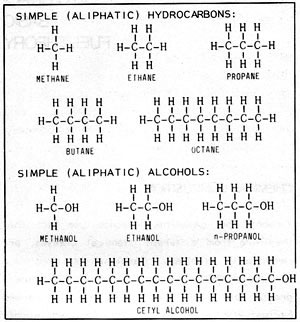
Alcohols can be thought of as hydrocarbons in which one of the hydrogen atoms has been replaced by a "hydroxyl group" which consists of a hydrogen atom bonded to an oxygen atom. Thus methane becomes the simplest alcohol, methanol. Ethane becomes ethanol, propane becomes propanol and so on. Like hydrocarbons, there are many alcohols of ever increasing complexity.
COMBUSTION PROPERTIES
One of the most important properties of a fuel is the amount of energy obtained from it when it is burned. Referring to Figure 2-2, note that the hydrocarbon octane, which represents an "ideal" gasoline, contains no oxygen. In comparison, all of the alcohols contain an oxygen atom bonded to a hydrogen atom in the hydroxyl radical. When the alcohol is burned, the hydroxyl combines with a hydrogen atom to form a molecule of water. Thus, the oxygen contained in the alcohol contributes nothing to the fuel value.
Figure 2-2: PHYSICAL PROPERTIES of ALCOHOL and GASOLINE
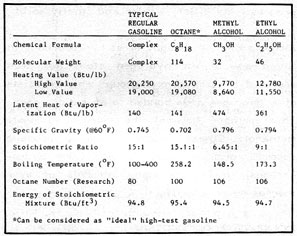
The relative atomic weights of the atoms involved are: hydrogen, 1 ; carbon, 12; and oxygen, 16. Since methyl alcohol has an atomic weight of 32, half the molecule cannot be "burned" and does not contribute any fuel value. As expected, methanol has less than half the heat value (expressed in Btu/lb) of gasoline. Ethanol, with 35% oxygen, is slightly better with 60% of the heat value of gasoline.
If the heating value of methyl and ethyl alcohol were considered alone, they would appear to be poor choices as motor fuels. However, other redeeming qualities such as "latent heat of vaporization" and anti-knock values make alcohol fuels superior, in some ways, to gasoline.
When a fuel is burned, a certain amount of air is required for complete combustion. When the quantity of air and the quantity of fuel are exactly balanced, the fuel air mixture is said to be "stoichiometrically" correct. Again referring to Figure 2-2, the stoichiometric ratio for gasoline is 15:1 or 15 pounds of air for each pound of gasoline. The figures for methyl and ethyl alcohol are 6.45:1 and 9:1 respectively. On a practical level, this means that to burn alcohol effectively, the fuel jets in the carburetor must be changed or adjusted to provide 2.3 pounds of methanol or 1.66 pounds of ethanol for each 15 pounds of air.
Referring to the last entry in Figure 2-2, an interesting fact is that if we provide the correct stoichiometric mixture and then compare on the basis of the energy (in Btu's) contained in each cubic foot of the different fuel/air mixtures, the fuels are almost identical: gasoline 94.8 Btu per cubic foot; methanol 94.5 and ethanol 94.7! This means that gasoline and alcohol are about equal in what is called "volumetric efficiency" when burned in a correctly adjusted engine.
VOLATILITY
Another important quality in a motor fuel is "volatility", or the ability to be vaporized. As previously noted, methyl alcohol contains less than half the heat value of gasoline and ethyl alcohol contains only about 60%. The next higher alcohol, propyl alcohol with three carbon atoms, contains only 26.6% oxygen and thus about 74% of the heat value of gasoline. It is apparent that the more complex the alcohol, the closer its heat value comes to that of gasoline. Cetyl alcohol (Figure 2-1), for example, contains only about 6.6% oxygen and thus has about 90% of the heat value of gasoline. However, this alcohol is a solid wax! It can't be conveniently vaporized and mixed with air in an engine and so is useless as a motor fuel. Consequently, in considering alcohol fuels, a compromise must be made between heat value and volatility.
Closely related to volatility is a quality called "latent heat of vaporization". When a liquid is at its boiling point, a certain amount of additional heat is needed to change the liquid to a gas. This additional heat is the latent heat of vaporization, expressed in Btu/lb in Figure 2-2. This effect is one of the principles behind refrigeration and the reason that water evaporating from your skin feels cool.
Referring to Figure 2-2, gasoline has a latent heat of about 140 Btu/lb; methanol, 474 Btu/lb; and ethanol, 361 Btu/lb. In an engine, vaporization of the gasoline fuel/air mixture results in a temperature drop of about 40 degrees Fahrenheit. Under similar conditions, the temperature drop for ethyl alcohol will be more than twice that of gasoline, and for methanol the drop will be over three times as great. These temperature drops result in a considerably greater "mass density" of the fuel entering the engine for alcohol as compared to gasoline. The result is a greatly increased efficiency for alcohol fuels. To visualize why, remember that at a given pressure, the amount of space a gas occupies is directly proportional to the temperature. For example, if one pound of a gas fits into a certain container at a given pressure and the temperature is cut in half, the container will now hold two pounds of the gas at the same pressure. In an engine, a stoichiometric mixture of methanol and air would be over three times colder than the same gasoline/air mixture. This means that there is now over three times (by weight) as much methanol in the cylinder. Now, even though methanol has only half the heat value of gasoline, the net gain in "volumetric mass efficiency" is over three times. So, for example, if the gasoline/air mixture in a given engine cylinder produces 100 Btu on each stroke, the same engine would produce 150 Btu per stroke with methanol. This power gain due to increased volumetric mass efficiency is the primary reason for the popularity of methyl alcohol as a racing fuel. With ethanol the effect isn't quite as dramatic, but the greater heat value partially offsets the lower latent heat. Overall, this power increase with alcohol fuels considerably mitigates the liability of low heat value.
However, the increased cooling due to latent heat sometimes creates a problem in an engine converted to run on alcohol. Once vaporized, a certain amount of heat is required to keep the fuel from condensing back to the liquid state before it reaches the cylinder. To accomplish this, an engine is designed to provide this heat to the intake manifold. Alcohol, because of its greater latent heat, requires more heat than gasoline. This is one of the reasons that racing engines have short path manifolds and multiple carburetors. The shorter the distance the fuel must travel to the cylinder, the less chance of condensation and fuel distribution problems. On a practical level, most engines that have been converted to alcohol supply enough heat once they are warmed up. The main problem, as with high performance racing engines, is in starting a cold engine. This problem and the related fuel distribution problem will be discussed later in more detail.
OCTANE RATINGS
If a certain fuel is burned in an engine in which the compression ratio can be varied and this ratio is gradually increased, a point will be reached when the fuel will detonate prematurely. This is because as a gas is compressed, heat is generated. If the explosive fuel/air mixture in an engine cylinder is compressed enough, the resulting heat will cause it to detonate. Since gasoline engines are designed so that the mixture is detonated by the spark plug at the beginning of the downward movement of the piston following the compression stroke, preignition or "knock" occurring during the compression stroke is undesirable. Indeed, severe knock can quickly overstress and destroy an engine.
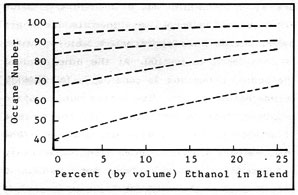
Since greater compression ratios in an engine mean increased power per stroke and greater efficiency, the ability of a fuel to resist premature detonation is a desirable quality. The "octane" numbers assigned to fuels are based on the pure hydrocarbon, octane, which is considered to be 100. At the other end of the scale, n-heptane is considered to have an octane rating of zero. The octane number of an unknown fuel is based on the percentage volume of a mixture of octane and n-heptane that matches it in preignition characteristics. In practice, these tests are conducted in a special test engine with variable compression. As noted in Figure 2-2, alcohols have a relatively high anti-knock or octane rating. As noted in Figure 2-3, alcohols have the ability to raise considerably the octane ratings of gasolines with which they are mixed. The effect is greatest on the poorer grades of gasoline. A 25% blend of ethanol and 40 octane gasoline will have a net increase of almost 30 points! This increase is one of the major advantages of "gasohol". The ability to increase octane rating means that: (1) a lower (therefore cheaper) grade of gasoline can be used to obtain a fuel with a certain octane rating; and (2) the use of traditional pollution producing anti-knock additives such as tetraethyl lead can be eliminated. The addition of about 10-15% ethanol to unleaded gasoline raises the octane rating enough so that it can be burned in high compression engines that previously could not use unleaded fuel. This use of ethanol is not new, of course, because ethanol was the original gasoline additive for increasing the octane rating. The term "ethyl" used to describe a high-test gasoline comes from ethyl alcohol, not tetraethyl lead!
Figure 2-3: OCTANE INCREASE of ALCOHOL/GASOLINE BLENDS
WATER INJECTION
During World War II, the military made extensive use of water injection in high performance piston aircraft engines. Later, water injection was used by both civilian and military jet aircraft to provide extra thrust, principally on takeoff. Even today, water injection systems are available that can be installed in automobiles. The fact is that, within certain limits, these systems actually do increase power. Referring back to Figure 2-2, note that the latent heat of vaporization for gasoline is about 140 Btu/lb and for ethanol about 361 Btu/lb. Water has a latent heat of about 700 Btu/lb! Therefore, if a little water is injected into the carburetor in the form of an ultra-fine mist, the latent heat of the water will cool the charge and increase volumetric efficiency. In addition, when the charge is fired in the cylinder, the water will turn to high-pressure steam and provide additional power due to the pressure exerted by the steam. There are definite limits, however, to the amount of water that can be injected. Too much will cause excessive cooling and misfiring.
The use of water injection with a gasoline fueled engine requires a separate metering and injection system because water and gasoline do not mix. Ethanol and water, however, do mix and the benefits of water injection can be had simply by adding the desired amount of water to the alcohol in the fuel tank.
EXHAUST COMPOSITION
In theory, a hydrocarbon fuel when burned should produce only water and carbon dioxide (CO2 ) as exhaust gases. Carbon dioxide, of course, is completely non-poisonous being the gas we exhale when we breathe, the bubbles in carbonated beverages, and the gas plants turn back into oxygen during the photosynthesis cycle.
However, such ideal combustion rarely occurs even in the most perfectly adjusted engine. What is actually produced is a large amount of poisonous carbon monoxide (CO) and other complex (and undesirable) emissions arising from impurities like sulfur and additives such as lead or phosphorus.
Pure alcohol when burned under ideal conditions also produces, in theory, only carbon dioxide and water. Again, in practice, varying amounts of carbon monoxide are also produced. However, the amounts of carbon monoxide are usually much lower than with gasoline. In addition, alcohol fuel will contain no sulfur and no additives, and will not produce the related, undesirable combustion by-products. Pure alcohol fuels are extremely clean burning.
Many studies have been made to determine whether alcohol/gasoline blends have any positive effect on emissions. In general, the data show that no great changes occur in blends of 20% or less. What happens is simply that in a 10% alcohol/gasoline blend, for example, about 10% of the gasoline emissions are replaced with alcohol emissions. Since alcohol does burn considerably cleaner, the amount of emission improvement is proportional to the amount of alcohol in the blend.
Pure alcohol, as an anti-pollution fuel, would easily meet and exceed all emission requirements without the need for exotic and costly exhaust plumbing and catalytic converters. With alcohol blends, the chief advantage would be in the use of ethanol to replace lead and other undesirable compounds used to raise the octane number.
ENGINE PERFORMANCE - STRAIGHT ALCOHOL
Having looked at a few of the basic factors which influence the performance of fuels in an engine, let us now examine some actual engine tests. Figure 2-4 is a plot of 198 proof (99%) ethyl alcohol as compared to gasoline. "Mean Effective Pressure" in the graph is a direct indication of the power produced. The increased mean effective pressure (M.E.P.) of alcohol at all mixture ratios is the most noticeable difference between the two fuels. This increase in M.E.P. is due mainly to the greater volumetric efficiency that results from the high latent heat of vaporization of ethanol and the resulting greater mass density of the fuel/air mixture.
Figure 2-4: ENGINE PERFORMANCE of ETHANOL vs GASOLINE
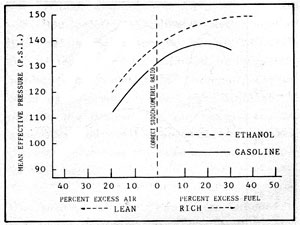
Note that the M.E.P. of ethanol increases with mixtures having up to 40% excess fuel, whereas for gasoline, the maximum pressure is reached at 20% excess fuel. It would seem that to achieve maximum power from an alcohol-burning engine there would be a temptation to burn very rich mixtures. Fuel economy aside, it should be noted that the rich mixtures necessary to obtain maximum M.E.P. are accompanied by incomplete burning of the fuel and the resultant lowering of overall thermal efficiency. The lean limits for alcohol and gasoline, therefore, are about the same, and both fuels develop maximum thermal efficiency at about 15% excess air. With mixtures leaner than 15% both fuels loose thermal efficiency.
Figure 2-5: HORSEPOWER COMPARISON of ETHANOL vs GASOLINE
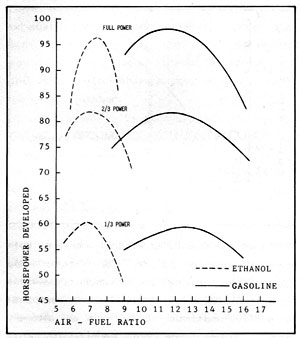
Figure 2-5 compares engine horsepower and air/fuel ratios for ethanol and gasoline in a six cylinder engine. The fuels in this case were 190 proof (95%) ethanol and "regular" gasoline having a specific gravity of 0.745. In the tests, air was supplied to the intake manifold at a constant 100 degrees Fahrenheit, and the carburetor needle valve was adjusted to provide the desired fuel/air ratios. The 2/3 and 1/3 loads were established by adjusting the throttle to give the same manifold pressure for both fuels.
The smaller air/fuel ratios for ethanol in comparison with gasoline are evident. In this test with the air supplied at the same temperature for both fuels, the correct fuel/air mixture should produce about 2% more power from gasoline than ethanol. However, alcohol, with its greater latent heat, requires more manifold heat to remain completely vaporized. In another test where this additional heat was supplied, the correct alcohol/air mixture gave 8.6% more power with ethanol! Note also that the test depicted in Figure 2-5 was run with alcohol that contained 5% water. This benefit of water injection probably inflated the alcohol power results to a certain degree. However, the main point illustrated is that the two fuels are remarkably similar in performance in a correctly adjusted engine.
ENGINE PERFORMANCE - ALCOHOL BLENDS
Although alcohol blends can be made from both ethanol and methanol, the primary interest seems to be in the direction of ethanol. Methanol and gasoline have a limited miscibility (mixability) while ethanol and gasoline can be mixed in all proportions. Economic reasons also dictate the interest in ethanol since it is more readily made from renewable resources. In addition, ethanol is a slightly superior motor fuel alternative under most conditions.
Economics aside, a major advantage of blends is that up to a certain concentration (somewhere between 10 and 20%) they can be used with absolutely no modification of the engine.
Many studies on how the various blends affect engine performance are contradictory. The recent "Two Million Mile" test in Nebraska, claims slightly higher fuel economy. Other tests claim a slight decrease. Some tests claim slightly better emissions, others claim no significant change. In relation to power output, the tests are equally ambiguous. However, when all the data is sifted, the overall conclusion is that in the areas of fuel economy, emissions, and performance there just isn't any real difference.
Figure 2-3, as discussed under Octane Ratings, illustrates another major advantage of alcohol blends, namely the ability of alcohol to raise the anti-knock quality of the gasoline with which it is mixed. This means, of course, that lower, cheaper grades of gasoline can be used to obtain a fuel with the desired octane rating, and the use of pollution producing additives can be eliminated. This is a significant advantage from the economic standpoint because the manufacture of high-octane blending stocks is expensive. Also, as previously mentioned, it is possible to raise the octane rating of unleaded gasoline so that it can be used in engines that previously required high-test leaded gasoline.
Alcohol blends do have one relatively minor drawback. The presence of even small amounts of water in the blend will cause a portion of the alcohol and gasoline to separate. At room temperature, less than 1% water can do the damage. As the temperature is lowered, amounts as small as 0.01% can cause separation. However, various substances such as benzene (benzol), acetone, and butyl alcohol can be added to the blend to increase water tolerance. Closed fuel systems, now in use, prevent moisture from forming inside the gas tank. Oil companies, given the proper incentive, could dry out their storage facilities and pipelines. Also, extensive use of alcohol blends over the past 50 years is ample evidence that the problem can be solved.
Chapter Index
Chapter 1 AN OVERVIEW
Chapter 2 BASIC FUEL THEORY
Chapter 3 UTILIZATION OF ALCOHOL FUELS
Chapter 4 ETHANOL PRODUCTION - GENERAL DISCUSSION
Chapter 5 PROCESSING STEPS COMMON TO ALL MATERIALS
Chapter 6 PROCESSING STEPS SPECIFIC TO SACCHARINE MATERIALS
Chapter 7 PROCESSING STEPS SPECIFIC TO STARCHY MATERIALS
Chapter 8 PROCESSING STEPS SPECIFIC TO CELLULOSE MATERIALS
Chapter 9 YEAST AND FERMENTATION
Chapter 10 INDIVIDUAL RAW MATERIALS
Chapter 11 DISTILLATION
Chapter 12 DRYING THE ALCOHOL
Chapter 13 MASHING AND FERMENTATION EQUIPMENT
Chapter 14 DISTILLATION EQUIPMENT
Chapter 15 SOLAR STILLS
Chapter 17 PUTTING IT ALL TOGETHER
Chapter 18 THE FUTURE
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
