1 Background

Mr. L. John Fry beside a three-digester unit he made in 1973. Gas holder center, water-heater on the right. Buckets in the foreground were for loading raw materials. This unit was taken down in 1974.
Figures
(On my S. African farm):
Capital cost = $10,000
Gas per day = 8,000 ft3
Value, as gas = $7.57/day or $16,578 over six years
Value, as electricity = $7.43/day or $16,271 over six years
PLUS savings down from 8 man/days per week to 1 man/day. PLUS 5 tons Nitrogen, 4 1/2 tons Phosphates and 1 ton Potash per year in liquid end products.
When organic material decays it yields useful by-products. The kind of by-product depends on the conditions under which decay takes place. Decay can be aerobic (with oxygen) or anaerobic (without oxygen). Any kind of organic matter can be broken down either way, but the end products will be quite different (Fig. 1).
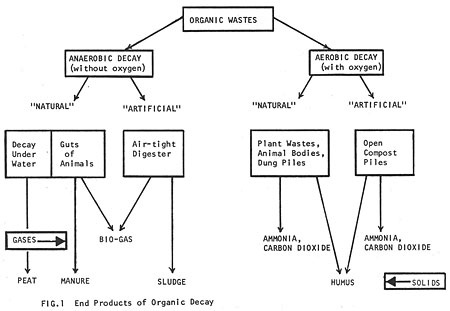
It is possible to mimic and hasten the natural anaerobic process by putting organic wastes (manure and vegetable matter) into insulated, air-tight containers called digesters. Digesters are of two types:
- Batch-load digesters which are filled all at once, sealed, and emptied when the raw material has stopped producing gas; and
- Continuous-load digesters which are fed a little, regularly, so that gas and fertilizer are produced continuously.
The digester is fed with a mixture of water and wastes, called "slurry." Inside the digester, each daily load of fresh slurry flows in one end and displaces the previous day's load which bacteria and other microbes have already started to digest.
Each load progresses down the length of the digester to a point where the methane bacteria are active. At this point large bubbles force their way to the surface where the gas accumulates. The gas is very similar to natural gas and can be burned directly for heat and light, stored for future use, or compressed to power heat engines.
Digestion gradually slows down toward the outlet end of the digester and the residue begins to stratify into distinct layers (Fig. 2).
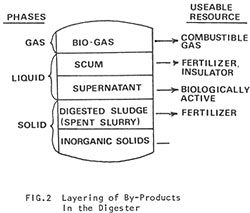
- Sand and Inorganic Materials at the bottom.
- Sludge, the spent solids of the original manure reduced to about 40% of the volume it occupied in the raw state. Liquid or dry sludge makes an excellent fertilizer for crops and pond cultures.
- Supernatant, the spent liquids of the original slurry. Note that the fertilizing value of the liquid is as great as sludge, since the dissolved solids remain.
- Scum, a mixture of coarse fibrous material, released from the raw manure, gas, and liquid. The accumulation and removal of scum is one of the most serious problems with digesters. In moderate amounts, scum can act as an insulation. But in large amounts it can virtually shut down a digester.
For perspective, consider the total fuel value of methane that could be produced from the available organic wastes in the United States.
Total Fuel Value of U.S. Methane Resources Supplied by Digestion of Readily Collectable, Dry, Ash-Free Organic Wastes.
I. Fuel Value of U.S. Methane Resources (From Ref. 1)
A. Organic wastes in U.S./year
800 million tons (dry weight)
B. Dry organic waste readily collectable
C. Methane available from "B"
D. Fuel value of methane from "C"
II. Fuel Consumption of U.S. Farm Equipment (From Ref. 2)
A. Total gasoline consumed (1965)
B. Total energy consumed by "A"
III. Total U.S. Natural Gas Consumption (1970)
IV. Total U.S. Energy Consumption (1970)
*Urban refuse; higher figure for manure and agricultural wastes.
So, speaking generally, methane gas converted from easily available organic wastes could supply about 150% of the gasoline energy used by all U.S. farm equipment (1965), 7% of the 1970 natural gas energy, and 2% of the total 1970 U.S. energy demands.
Methane-Gas Plant: Synergy at Work
When we consider digesters on a homestead scale, there are two general questions to ask: (1) with the organic wastes and resources at hand, what kind of digester should be built, and how big should it be? and (2) what is the best way of using the gas and sludge produced to satisfy the energy needs of the people involved? (whether the sludge should be used to fertilize crops, fish or algae ponds, and whether the gas should be used directly for heat, and light, or stored, or fed back to the digester to heat it, etc. Fig. 3).
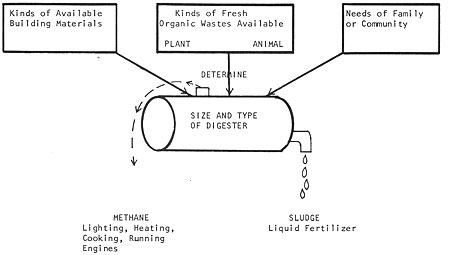
Fig. 3 Related considerations of a digester operation
The first question involves the digester itself, which is just the heart of a whole energy system. The second question is synergistic; you can choose which products are to be generated by digestion and how to use them or feed them back to the digester, creating an almost endless cycle if you wished (Fig. 4).
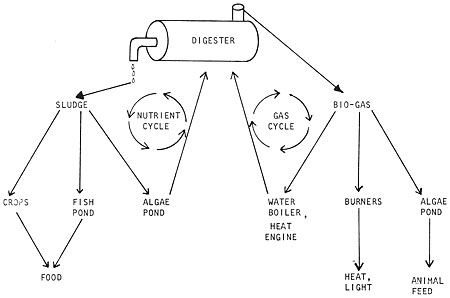
Fig. 4 The closed nutrient system of a complete digester operation
The model in Fig. 4 is idealized from oriental aquaculture systems and other ideas, both old and new. A single pathway can be developed exclusively (have your digester produce only sludge to feed an algae pond) or you can develop the potential synergy (many possible systems working together as an integrated whole, Fig. 5).
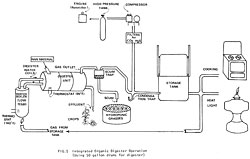
Fig. 5 Integrated organic digester operation (using 50 gallon drums for digester) -- Bigger image
The small farmer or rural homesteader can take a step toward ecological self-sufficiency by producing some of his fuel and fertilizer needs using a digester to convert local wastes.
Total dependence on conventional fuels, especially in rural areas, is likely to become a serious handicap in the years to come as reserve shortages and specialized technologies hike the costs of fossil and nuclear fuels. But by producing energy from local resources, it is possible to be partially freed from remote sources of increasingly expensive fuel supplies.
2 History
In nature, anaerobic decay is probably one of the earth's oldest processes for decomposing wastes. Organic material covered by a pool of warm water will first turn acid and smell rank, then slowly over about six months will turn alkali. The methane bacteria, always present, will take over and decompose it, and gas bubbles will rise to the surface.
Anaerobic decay is one of the few natural processes that hasn't been fully exploited until recent times. Pasteur once discussed the possibilities of methane production from farmyard manure. And (according to a report issued from China, April 26, 1960) the Chinese have used "covered lagoons" to supply methane fuel to communes and factories for decades. But the first attempt to build a digester to produce methane gas from organic wastes (cow dung) appears to have been in Bombay, India in 1900. At about this time, sewage plants started digesting sewage sludge in order to improve its quality. This started a mass of laboratory and small-scale experiments during the 20's and 30's (many of them summarized by Acharya, Ref. 3).
During World War II, the shortage of fuel in Germany led to the development of methane plants in rural areas, where the gas was used to power tractors. The idea spread into Western Europe, until fossil fuels once again became available (although, today, many farmers in France and Germany continue to use home digesters to produce their own methane fuel gas).
Currently the focus of organic digester/bio-gas research is in India. India's impetus has been the overwhelming need of a developing country to raise the standard of living of the rural poor. Cows in India produce over 800 million tons of manure per year; over half of this is burned for fuel and thus lost as a much needed crop fertilizer. (Ref. 4) The problem of how to obtain cheap fuel and fertilizer at a local level led to several studies by the Indian Agricultural Research Institute in the 1940's to determine the basic chemistry of anaerobic decay. In the 1950's, simple digester models were developed which were suitable for village homes. These early models established clearly that bio-gas plants could:
- provide light and heat in rural villages, eliminating the need to import fuel, to burn cow dung, or to deforest land;
- provide a rich fertilizer from the digested wastes; and
- improve health conditions by providing air-tight digester containers, thus reducing disease borne by exposed dung.
More ambitious designs were tested by the Planning Research and Action Institute in the late 1950's. Successes led to the start of the Gobar Gas Research Station at Ajitmal where, with practical experience from the Khadi and Village Industries Commission, two important pamphlets (Ref. 5, 6) were published on the design of village and homestead "bio-gas" plants in India.
In America, where the problem is waste disposal, rather than waste use, organic digesters have been limited to sewage treatment plants. (Ref. 7, 8.) In some cases sludge is recycled on land or sold as fertilizer (Ref. 9, 10), and methane gas is used to power generators and pumps in the treatment plants (Ref. 11). The Hyperion sewage treatment plant in Los Angeles generates enough methane from its primary treatment alone to power its 24-2,000 hp. diesel engines. Usually, however, both sludge and gas are still regarded as waste problems.
Much information on digestion and small-scale digester operations comes from experiences in India, Western Europe and South Africa and journals such as: Compost Science, Water Sewage Work, Soils and Fertilizer, Waste Engineering, Sewage and Industrial Wastes and recent publications of the U.S. Environmental Protection Agency and Solid Waste Conferences (see Bibliography at end). An excellent book to learn from is called: Manual of Instruction for Sewage Plant Operators, put out by the New York State Dept. of Health and available from the Health Education Service, P.O. Box 7283, Albany, New York 12224.
A great deal of information can be found in pre-WW II sewage journals, especially Sewage Works Journal. After WW II, as with most other kinds of science and technology, waste treatment research became a victim of the trend to make machines ever bigger, and information increasingly incomprehensible.
3 Biology of Digestion
Bio-Succession in the Digester
Perhaps the most important thing to remember is that digestion is a biological process.
The "anaerobic" bacteria responsible for digestion can't survive with even the slightest trace of oxygen. So, because of the oxygen in the manure mixture fed to the digester, there is a long period after loading before actual digestion takes place. During this initial "aerobic" period, traces of oxygen are used up by oxygen-loving bacteria, and large amounts of carbon dioxide (C02) are released.
When oxygen disappears, the digestion process can begin. That process involves a series of reactions by several kinds of anaerobic bacteria feeding on the raw organic matter. As different kinds of these bacteria become active, the by-products of the first kind of bacteria provide the food for the other kind (Fig. 6). In the first stages of digestion, organic material which is digestible (fats, proteins and most starches) are broken down by acid producing bacteria into simple compounds. The acid bacteria are capable of rapid reproduction and are not very sensitive to changes in their environment. Their role is to excrete enzymes, liquefy the raw materials and convert the complex materials into simpler substances (especially volatile acids, which are low molecular weight organic acids -- See 4 Raw Materials). The most important volatile acid is acetic acid (table vinegar is dilute acetic acid), a very common by-product of all fat, starch and protein digestion. About 70% of the methane produced during fermentation comes from acetic acid (Ref. 12).
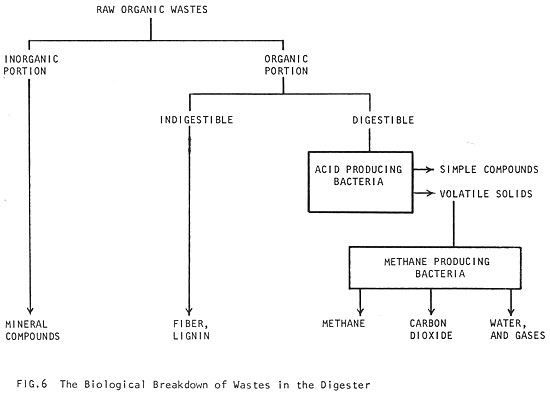
Once the raw material has been liquefied by the acid producing bacteria, methane producing bacteria convert the volatile acids into methane gas. Unlike the acid bacteria, methane bacteria reproduce slowly and are very sensitive to changes in the conditions of their environment. (More information on the biology of methane fermentation can be found in Ref. 13 and 14.)
Biologically, then, successful digestion depends upon achieving and (for continuous-load digesters) maintaining a balance between those bacteria which produce organic acids and those bacteria which produce methane gas from the organic acids. This balance is achieved by a regular feeding with enough liquid (see 4 Raw Materials) and by the proper pH, temperature and the quality of raw materials in the digester.
pH and the Well-Buffered Digester
To measure the acid or alkaline condition of a material, the symbol "pH" is used. A neutral solution has pH = 7; an acid solution has pH below 7; and an alkaline solution has pH above 7. The pH has a profound effect on biological activity, and the maintenance of a stable pH is essential to all life. Most living processes take place in the range of pH 5 to 9. The pH requirements of a digester are more strict (pH 7.5-8.5, Fig. 7).
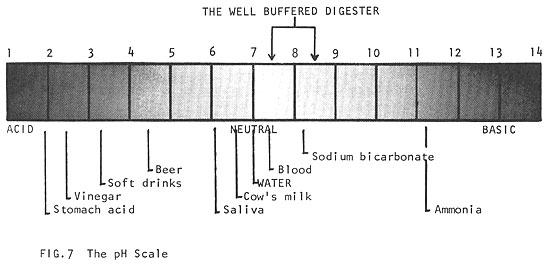
During the initial acid phase of digestion, which may last about two weeks, the pH may drop to 6 or lower, while a great deal of CO2 is given off. This is followed by about three months of a slow decrease in acidity during which volatile acids and nitrogen compounds are digested, and ammonia compounds are formed (this ammonia becomes important when we consider the fertilizer value of sludge). As digestion proceeds, less CO2 and more methane is produced and the pH rises slowly to about 7. As the mixture becomes less acid, methane fermentation takes over. The pH then rises above the neutral point (pH = 7), to between pH 7.5 and 8.5. After this point, the mixture becomes well buffered; that is, even when large amounts of acid or alkali are added, the mixture will adjust to stabilize itself at pH 7.5 to 8.5.
Once the mixture has become well buffered, it is possible to add small amounts of raw material periodically and maintain a constant supply of gas and sludge (continuous load digesters). If you don't feed a digester regularly (batch-load digesters), enzymes begin to accumulate, organic solids become exhausted and methane production ceases.
After digestion has stabilized, the pH should remain around 8.0 to 8.5. The ideal pH values of effluent in sewage treatment plants is 7 to 7.5, and these values are usually given as the best pH range for digesters in general. From our experience, a slightly more alkaline mixture is best for digesters using raw animal or plant wastes.
You can measure the pH of your digester with "litmus" or pH paper which can be bought at most drug stores. Dip the pH paper into the effluent as it is drawn off. Litmus paper turns red in acid solutions (pH 1 to 7) and blue in alkaline solutions (pH 7 to 14). You can get more precise measurements using pH paper which changes colors within a narrow range of pH values.
Problems with pH.
Condition
Too acid
(pH 6 or less)1) Adding raw materials too fast
Reduce feeding rate; Ammonia
2) Wide temperature fluctuation
Stabilize temperature
3) Toxic Substances
-
4) Build-up of scum
Remove scum
Too Alkaline
(pH 9 or more)1) Initial raw material too alkaline
Patience
Never put acid into digester
If the pH in the continuous-load digester becomes too acidic (Table 2), you can bring it up to normal again by adding fresh effluent to the inlet end, or by reducing the amount of raw material fed to the digester, or as a last resort, by adding a little ammonia. If the effluent becomes too alkaline, a great deal of C02 will be produced, which will have the effect of making the mixture more acidic, thus correcting itself. Patience is the best "cure" in both cases. NEVER add acid to your digester. This will only increase the production of hydrogen sulfide.
Temperature
For the digesting bacteria to work at the greatest efficiency, a temperature of 95°F (35°C) is best. Gas production can proceed in two ranges of temperature: 85-105°F (29-40°C) and 120-140°F (49-60°C). Different sets of acid-producing and methane bacteria thrive in each of these different ranges. Those active in the higher range are called heat-loving or "thermophilic" bacteria (Fig. 8). Some raw materials, like algae, require this higher range for digestion. But digesters are not commonly operated at this higher range because:
- most materials digest well at the lower range,
- the Thermophilic Bacteria are very sensitive to any changes in the digester,
- the sludge they produce is of poor fertilizer quality, and
- it is difficult to maintain such a high temperature, especially in temperate climates.
The bacteria that produce methane in the "normal range" 90-95°F (32-35°C) are more stable and produce a high quality sludge. It is not difficult to maintain a digester temperature of 95°F (35°C) (See 6 Digesters -- Heating Digesters).
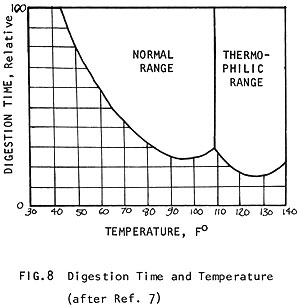
The same mass of manure will digest twice as fast at 95°F (35°C) than it will at 60°F (15°C) (Fig. 8) and it produces nearly 15 times more gas in the same amount of time! (Fig. 9) (See how the amount of gas produced improves with temperature to 80-100°F (27-38°C), where production is optimum.) In Fig. 10 it can be seen how a different amount of gas is produced when the digester is kept at 60°F (15°C) than when it is kept at 95°F (35°C).
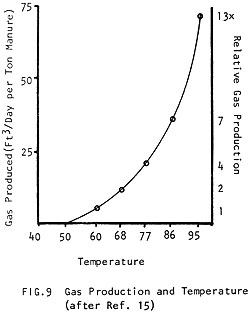
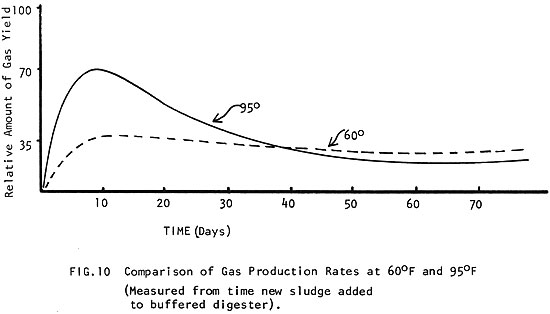
Next: 4 Raw Materials
12 References
Back to Table of Contents
Back to the Biofuels Library
Back to the Small Farms Library
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
