From E. Boullanger: Distillerie Agricole et Industrielle (Paris: Ballière, 1924).
Translation from the French by F. Marc de Piolenc (piolenc@reporters.net).
WE owe to Mr. Mariller some very remarkable studies on the industrial production of absolute alcohol. We have already noted, in our first volume (page 53), the industrial applications implemented by Mr. Mariller of the method of distillation of azeotropic mixtures discovered by Young in 1902. From 1920 on, Messrs Mariller and Coutant have used benzene and gasoline, which they injected into rectifiers fed with alcohol at 95 degrees, to directly produce the mixture of gasoline and absolute alcohol constituting the national standard motor fuel. At the base, a hydrocarbon/absolute alcohol mixture was extracted which, diluted with gasoline, provided the fuel, and at the head a stream which, when decanted, gave a dehydrated upper layer to be returned for treatment and a water-bearing lower layer to be distilled for recovery of the alcohol that it contained. If production of absolute alcohol was desired, then one need only fractionate it from its mixture with the hydrocarbon.
Later Mr. Mariller acknowledged that it is much simpler to operate in two phases: a first column eliminates water and the second, receiving the dehydrated product, yields at the head a mixture that is returned to the first column, and at the tails absolute alcohol. With everything figured in, with appropriate recovery, the steam consumption dropped to 200 kilograms per hectoliter, while Young's batch method led, with recycling, to an expenditure of at least 600 kilograms.
But according to Mr. Mariller, the use of water-absorbing substances instead of alcohol-absorbing substances [for separating alcohol/water mixtures by absorption] must necessarily be more economical, for with alcohol-absorbents water, the third substance [absorbent] and the alcohol/substance mixture must all be evaporated and the mixture subsequently fractionated to recover pure alcohol. This results in additional evaporation which penalizes the overall cost of the method. Contrarily, with water absorbents, only water and a little entrained alcohol (if any) must be subsequently evaporated; steam consumption then falls to 30 kg per hectoliter of alcohol, or approximately frs 0.40 at the current [1924] price of coal.
These considerations led Mr. Mariller to his absolute alcohol production process by dehydration using glycerine. Alcoholic vapors passing through pure glycerine yield 99.2 alcohol directly, and merely adding potassium carbonate, for example, to the glycerine is sufficient for easily obtaining 99.8. The glycerine and the salt that it holds in solution are regenerated and returned to the circuit.
In what follows we will examine the apparatus that can be used in various situations, but a special analysis should be considered necessary for each factory, to make use of existing equipment.
Continuous rectifier case
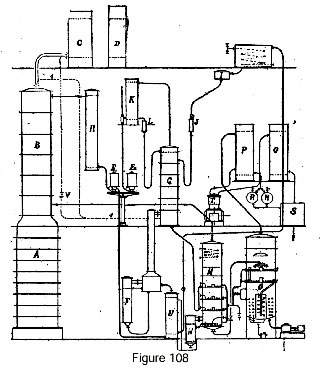 Let us imagine first a continuous rectifier (fig. 108) with its rectifying column B, its condensers C and D, and column A acting as a stripping column for the reflux of column B. Alcohol at 96.5 is received, after cooling in cooler R, in gauge E1.
Let us imagine first a continuous rectifier (fig. 108) with its rectifying column B, its condensers C and D, and column A acting as a stripping column for the reflux of column B. Alcohol at 96.5 is received, after cooling in cooler R, in gauge E1.
To transform this alcohol into absolute alcohol, it is allowed to run continuously into the recuperator U where it is heated, then it passes into the steam-heated evaporator F where it is entirely evaporated. The alcohol vapor thus obtained passes through column G, which is fed glycerine by a float chamber I and a flow meter J. The dehydrating liquid is contained in a feeder tank shown in the drawing, and can be readily heated or cooled by a serpentine heat exchanger coil.
The dehydrated alcohol vapor escapes from column G to a condenser K, and the anhydrous alcohol is received in gauge E2. To compensate for the heating effect produced by the absorption of water in column G, this reflux can be channeled to the feed plate itself, or better to a segment set above the feed plate, in order to retain any absorbing liquid that is accidentally entrained.
If we wish to directly connect the vapor from the rectifying column B to the concentrating column G, it is only necessary to provide the pipe I shown dotted in the drawing, and accessory piping connecting this pipe to condenser C, with a regulating cock V inserted between them. Inasmuch as the resistance to the passage of vapor through column G is greater than that of condensers C and D, merely opening this cock more or less is enough to automatically regulate the reflux from condensers C and D. This reflux is not shown in the drawing; it is usually brought back to the top of column B, without modification.
The dehydrated liquid collected at the base of column G flows into column M, formed by stacked plates having steam heating coils. In addition, the base of this column is heated by a serpentine coil or tubular heater N. The vapor that is released, consisting of water and alcohol retained by the adsorbent, return directly to the rectifying column B.
To avoid entrainment, a serpentine condenser is used, located in the upper part of column M, or a condenser of any other type whose condensate is collected in washing plates in which the vapor bubbles up before escaping to rectifying column B.
In many cases, and notably when the intent is to produce alcohol that is not completely anhydrous, the liquid obtained at the base of column M can be directly taken up by a pump and sent to the feeder tank. On the other hand, to obtain alcohol at 99.8-100 percent with glycerine with dehydrating salts added, it is vital to complete the total dehydration of the product, an operation which can only be accomplished under vacuum to avoid any degradation of the absorbent.
Concentration under vacuum must be carried out on a liquid that is completely free of alcohol, in order to avoid alcohol losses which always take place in vacuo when operating on a liquid that contains any appreciable quantity of it.
It has been recognized that total alcohol extraction can be accomplished in column M by completing the heating process with a slight direct injection of steam in the sub-base of the column.
From column M, the liquid passes automatically into a concentrator O which, being under vacuum, provides suction.
Upon arrival, the liquid undergoes evaporation; it is then concentrated in stacked plates similar to those of column M, and finally in a sub-base chamber equipped with powerful coils in which steam flows under pressure.
The liquid must be brought to a temperature such that it is completely rid of water. For glycerine that is carbonated by the addition of potassium carbonate, the correct temperature is approximately 160 to 170 degrees. At the base of this column, the regenerated absorbing liquid is taken up by a pump and delivered to a tank for return to the circuit.
The upper portion of the concentrator is so constructed as to avoid any entrainment taking place. It can for instance be equipped with diffusers, with labyrinths or with a column with flow splitters, that column receiving a stream of a washing liquid for retaining entrained matter in solution.
Finally, the vapor passes to a condenser P, and the liquid is received in reservoirs R. This liquid must be extracted using a special pump.
The gases aspirated by vacuum pump T are delivered to a second condenser Q and all the liquid obtained is assembled in tank S. This liquid is water that is completely free of alcohol, but as it takes up very little volume it is perfectly possible, if we want to avoid any loss, however minimal, to add this liquid to the fermented mash to be distilled.
It is also possible to use part of the rectifying column for producing absolute alcohol, thus eliminating column G. In this case, the rectifying column B is partitioned into two stacked portions. The vapor from the lower segment goes to the condenser and returns to the base of the upper segment; the reflux from the condenser feeds the lower segment, and the upper segment is fed by a stream of absorbent liquid. The apparatus for regenerating the absorbent is identical to that described earlier.
Continuous direct rectifier case
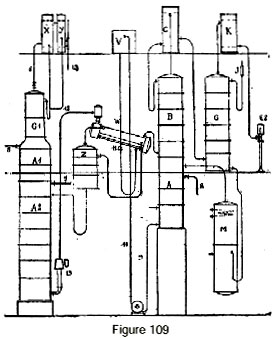
Figure 109 represents a continuous direct rectifier for fermented mash, directly producing absolute alcohol with the smallest possible expenditure of steam. In this apparatus, the liquid to be distilled enters through 5 into column A1, which is charged with removing head products more volatile than alcohol. The liquid then enters column A2 which is an extension of the preceding one, where it is completely stripped of alcohol. The alcohol vapor passes through pipe 7 into a concentrating column Z of a few plates. Another part of the vapor passes into column A1 and evaporates the head products which are concentrated in plates G1 and whose flow is regulated by a cock placed in drainpipe 6 of this column. They are then condensed in condensers X and Y.
The head products are then delivered to a special gauge via pipe 13.
The alcohol vapor, upon leaving column Z, passes into an evaporator W, which is fed pure water by a tank V and a regulating cock 11. In evaporator W, the alcohol vapor partially condenses while evaporating a certain quantity of water and thus supplying the vapor which will be taken up by thermo-compressor 13 to participate in the heating of column A2 and eventually of column A1.
The alcohol vapor, upon leaving the evaporator W, enter column AB whose vapor escapes to a mash preheater C. At the upper part of this column, the vapor arrives at a strength of approxi-mately 90-92 degrees; they are then directed to dehydration column G, which is supplied with the absorbing liquid.
The rest of the apparatus is identical to what was described earlier. The higher alcohols are extracted from column AB through one or more taps 8, in the usual fashion. Residual water obtained at the base of column A flows through pipe 9 to a pump that delivers it by pipe 10 to a tank V.
Adaptation of the Mariller-Granger process to an existing continuous direct rectifier
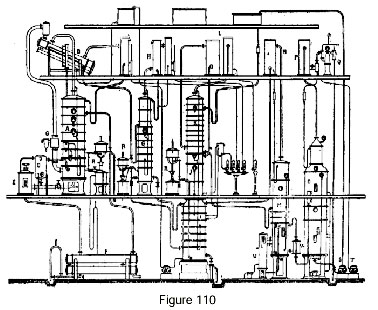
Figure 110 represents a rectifier of the usual type modified for the production of absolute alcohol.
Let us consider an existing apparatus comprised of: a distillation column A, a purifying column G and a rectification column IJ. The distillation column is equipped, as we saw earlier, for heat recovery by the use of a closed thermal circuit. For this purpose, the alcohol vapors escape to an evaporator B, which receives the spent distilled water coming from the rectifier I. This water, which arrives boiling in the evaporator, undergoes boiling there at a temperature of 85 degrees under partial vacuum. The excess water flows through a standpipe which drains it to a tank in such a way as to form an hydraulic trap. The steam for heating the column passes into a thermo-compressor G, which provides suction to aspirate the vapor from the evaporator and deliver it to the column, where it will contribute to heating. It is necessary, of course, to heat the beer especially with the residues from the distillation column or by any other means so as to free the greatest possible amount of heat at the evaporator. It is, however, possible to connect a small beer pre-heater for the vapor leaving the purifier.
Alcohol from the distillation column passes into the purifier, which eliminates ethers and aldehydes.
Finally, the alcohol passes into the rectifying column usually equipped with a condenser and a cooler L at its top. It is not necessary to obtain 96-degree alcohol from this column, and a lower strength is quite tolerable. Hence the top of the plates J is usually connected to the condenser by a closed plate and a lateral pipe. This condenser supplies the plates J with the necessary reflux. The usual extraction of higher alcohols occurs on plates I and J. The vapor, on leaving the condenser, is brought to the plates R, plates currently belonging to the rectifying column which are no longer receiving alcohol but a stream of glycerine coming from the feeder tank. The absolute alcohol leaving this column goes directly to a cooler and from there to the gauge.
The regeneration of the glycerine is obtained in two stages: 1st, alcohol stripping in column M; 2nd, complete dehydration in column O which operates under vacuum. A float chamber V automatically drains the alcohol-stripped liquid to introduce it into vacuum column O. The water vapor that escapes from the column is condensed in a condenser P, and from there the gasses pass to an ejector Q which provides vacuum for the entire plant. Water from the vacuum column is extracted through a hydraulic lock or by a pump T.
The regenerated glycerine absorbent is taken up by pump S which delivers it to a feeder tank.
Adaptation of the Mariller-Granger process to a batch rectifier
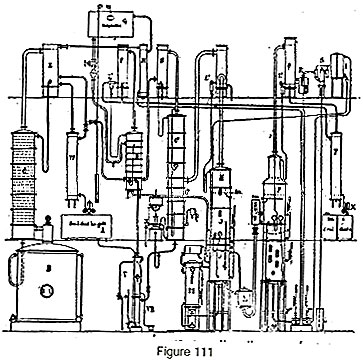
The batch rectifier (fig. 111) is represented by boiler B, column C, condenser E and cooler W. The production of absolute alcohol must obviously not take place using the bad-tasting fractions and the medium fractions, for we would then obtain an alcohol rich in aldehydes, ethers or higher alcohols. Further, it would not be suitable to run a batch process for absolute alcohol. To avoid these drawbacks, the absolute alcohol apparatus is calculated such that the good-tasting fraction produced over 24 hours can pass continuously into the part of the plant that dehydrates it. For this purpose, a tank is provided which receives a certain quantity of this alcohol while the good-tasting fraction is distilling over, so as to allow it to be evaporated in the evaporator V during the periods of the medium and bad-tasting fractions. To economize steam used in partially evaporating the alcohol, a recuperator VR is used which receives the residual boiling water from column C2. While the good-tasting fraction is coming over, a system of cocks allows the vapor to pass directly to the dehydration column D which receives the stream of absorbent liquid, which is stored in a feeder tank G. The cock connected to the column is fully open and the cock on cooler W is opened just enough to obtain the supplementary stream of good-tasting alcohol into tank A; this alcohol is treated during the medium- and bad-taste periods.
The absolute alcohol, upon leaving column D, is condensed in F, then sent to a cooler T and gauge X.
Regeneration of the absorbent is obtained as before in a system of two devices in series: 1st alcohol stripping (column JK, condenser L2 and tubular heater M); 2nd, concentration under vacuum with column OP condenser Q, vacuum tank R and ejector S.
In this apparatus, the alcohol obtained at the outlet of the alcohol stripping column could be condensed and returned in the liquid state to boiler B of the batch plant to have its strength increased, but we would thus lose the benefit of the latent heat of this vapor which allows it to be brought up to 95 degrees almost free of charge. In this case, it is enough to direct the vapor to a rectifying column C1 with stripper C2 and a condenser, upon leaving which the vapor goes to column D.
Application of the Mariller-Granger process to a high-proof column
When the plant already has a high-proof column, oil extraction has to be provided for in the lower plates if it doesn't already exist, and the apparatus must be completed with a purifier that the beer goes through after coming out of the preheater. The purified beer then enters the high-proof column. The vapors leaving the preheater are directed to the column for dehydration by glycerine, whose regeneration is accomplished by the components described earlier.
Economy of the method
Dehydration by glycerine gives rise to extreme reductions in reflux. Mr. Mariller has shown for example that one can proceed from vapor at
65 GL to 99.5 with 3.7 reflux
83 GL to 99.8 with 2.85 reflux
93 GL to 99.9 with 0.53 reflux
95 GL to 99.9 with 0.35 reflux
96 GL to 99.9 with 0.27 reflux.
We thus arrive at absolute alcohol with much lower reflux rates than the current ones, and this result is obtained with only 12 plates instead of the 40 or more plates of current rectifiers.
In these circumstances, it is obvious that the cost of the process per hectoliter of alcohol will be highly variable, depending on the starting strength of the alcohol, disposition of the equipment, etc. The process does not result in any loss of alcohol, any loss of absorbent, any supplementary labor requirement. As for the expenditure of steam: from 45 degrees on it is less than 25 kilograms per hectoliter of alcohol, which corresponds to 4 kilograms of coal at 100 francs per tonne, or frs 0.40. Even counting in 1 franc per hectoliter to take into account small absorbent leaks through joints and slightly increased consumption of steam, and by adding in 2 francs per hectoliter for royalties, we reach a cost price of 3 francs. This cost can be considerably reduced in an apparatus that ensures a reduced reflux rate and uses a closed thermal circuit, with a thermo-compressor. We do, however, need to add in the amortization of the special equipment, which varies with plant capacity. For a plant capacity of 200 hectoliters, counting 200,000 francs for equipment, amortization over 100 days would give a figure of 10 francs per hectoliter. Total cost in this case, then, would be near 13 francs per hectoliter, which would still leave a daily profit of 400 francs based on an added value of 15 francs per hectoliter. After this very short amortization period, the cost would drop to 3 francs, which would still leave an additional 7 francs per hectoliter assuming that added value would drop to 10 francs.
Other processes for preparing absolute alcohol
Besides the Ricard-Allenet process and the Mariller-Granger process described above, we find the chemical dehydration processes that subject either alcoholic liquors or alcoholic vapors to the action of extremely hygroscopic substances forming fixed hydrates with water.
The principal substances employed for this purpose are quicklime, calcium chloride, potassium carbonate, etc. Deininger ran vapor leaving the rectifier into a cylinder containing calcium chloride. Wolkoff sent alcohol vapor into an apparatus charged with potassium carbonate; the vapor, in passing through this salt, gave up its water vapor, which in turn dissolved the salt. The saline solution obtained in this fashion was then concentrated in a Porion furnace to regenerate potassium carbonate.
The only method that survives today consists of dehydrating alcohol vapor by passage over quicklime. The lime dehydrates the alcohol, stops acids and ethers and allows absolute alcohol to be obtained with a loss of 2 to 3%.
Back to Biofuels Library
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
